Neat1 promotes acute kidney injury to chronic kidney disease by facilitating tubular epithelial cells apoptosis via sequestering miR-129-5p
- PMID: 35619557
- PMCID: PMC9552914
- DOI: 10.1016/j.ymthe.2022.05.019
Neat1 promotes acute kidney injury to chronic kidney disease by facilitating tubular epithelial cells apoptosis via sequestering miR-129-5p
Abstract
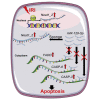
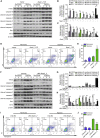
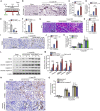
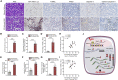
Acute kidney injury (AKI) is increasingly identified as a crucial risk factor for progression to CKD. However, the factors governing AKI to CKD progression remain largely unknown. By high-throughput RNA sequencing, we found that Neat1_2, a transcript variant of Neat1, was upregulated in 40-min ischemia/reperfusion injury (IRI), which resulted in the development of renal fibrotic lesions. The upregulation of Neat1_2 in hypoxia-treated TECs was attributed to p53 transcriptional regulation. Gain- and loss-of-function studies, both in vitro and in vivo, demonstrated that Neat1_2 promoted apoptosis of injured TECs induced by IRI and caused tubulointerstitial inflammation and fibrosis. Mechanistically, Neat1_2 shares miRNA response elements with FADD, CASP-8, and CASP-3. Neat1_2 competitively binds to miR-129-5p and prevents miR-129-5p from decreasing the levels of FADD, CASP-8, and CASP-3, and ultimately facilitates TEC apoptosis. Increased expression of Neat1_2 associated with kidney injury and TEC apoptosis was recapitulated in human AKI, highlighting its clinical relevance. These findings suggest that preventing TEC apoptosis by hindering Neat1_2 expression may be a potential therapeutic strategy for AKI to CKD progression.
Keywords: ESRD; acute kidney injury; apoptosis; chronic kidney disease; ischemic-reperfusion injury; long noncoding RNA; microRNA; renal fibrosis; tubular epithelial cell; tubulointerstitial fibrosis.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





Similar articles
-
Long non-coding RNA NEAT1 promotes lipopolysaccharide-induced injury in human tubule epithelial cells by regulating miR-93-5p/TXNIP axis.Med Microbiol Immunol. 2021 Jun;210(2-3):121-132. doi: 10.1007/s00430-021-00705-6. Epub 2021 Apr 22. Med Microbiol Immunol. 2021. PMID: 33885954
-
Exosomal miR-125b-5p deriving from mesenchymal stem cells promotes tubular repair by suppression of p53 in ischemic acute kidney injury.Theranostics. 2021 Mar 11;11(11):5248-5266. doi: 10.7150/thno.54550. eCollection 2021. Theranostics. 2021. PMID: 33859745 Free PMC article.
-
LncRNA NEAT1 promotes hypoxia-induced renal tubular epithelial apoptosis through downregulating miR-27a-3p.J Cell Biochem. 2019 Sep;120(9):16273-16282. doi: 10.1002/jcb.28909. Epub 2019 May 14. J Cell Biochem. 2019. PMID: 31090110
-
Non-coding RNAs in kidney injury and repair.Am J Physiol Cell Physiol. 2019 Aug 1;317(2):C177-C188. doi: 10.1152/ajpcell.00048.2019. Epub 2019 Apr 10. Am J Physiol Cell Physiol. 2019. PMID: 30969781 Review.
-
Metabolic Flexibility and Innate Immunity in Renal Ischemia Reperfusion Injury: The Fine Balance Between Adaptive Repair and Tissue Degeneration.Front Immunol. 2020 Jul 7;11:1346. doi: 10.3389/fimmu.2020.01346. eCollection 2020. Front Immunol. 2020. PMID: 32733450 Free PMC article. Review.
Cited by
-
Emerging roles of the long non-coding RNA NEAT1 in gynecologic cancers.J Cell Commun Signal. 2023 Sep;17(3):531-547. doi: 10.1007/s12079-023-00746-x. Epub 2023 Jun 13. J Cell Commun Signal. 2023. PMID: 37310654 Free PMC article. Review.
-
LncRNA-Top: Controlled deep learning approaches for lncRNA gene regulatory relationship annotations across different platforms.iScience. 2023 Oct 12;26(11):108197. doi: 10.1016/j.isci.2023.108197. eCollection 2023 Nov 17. iScience. 2023. PMID: 37965148 Free PMC article.
-
The function of miRNAs in the process of kidney development.Noncoding RNA Res. 2023 Aug 23;8(4):593-601. doi: 10.1016/j.ncrna.2023.08.009. eCollection 2023 Dec. Noncoding RNA Res. 2023. PMID: 37680850 Free PMC article. Review.
-
ANRIL, H19 and TUG1: a review about critical long non-coding RNAs in cardiovascular diseases.Mol Biol Rep. 2023 Dec 28;51(1):31. doi: 10.1007/s11033-023-09007-x. Mol Biol Rep. 2023. PMID: 38155319 Review.
-
Identification of lncRNA-miRNA-mRNA ceRNA network as biomarkers for acute kidney injury.Am J Transl Res. 2023 Sep 15;15(9):5730-5746. eCollection 2023. Am J Transl Res. 2023. PMID: 37854219 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

