Durable response after the discontinuation of pembrolizumab treatment due to an adverse event in a patient with advanced endometrial cancer: A case report
- PMID: 35619629
- PMCID: PMC9115643
- DOI: 10.3892/etm.2022.11336
Durable response after the discontinuation of pembrolizumab treatment due to an adverse event in a patient with advanced endometrial cancer: A case report
Abstract
The persistence of antitumor effects has been reported after the completion of treatment with immune checkpoint inhibitors (ICIs) for various types of carcinoma, such as malignant melanoma, exhibiting a durable response. A durable response has also been noted after the discontinuation of treatment at an early stage due to adverse events, including in renal pelvic cancer, pancreatic cancer and intrahepatic cholangiocarcinoma; however, to the best of our knowledge, a similar case report has not yet been published in the malignant gynecological tumor field. The present study described a patient with refractory advanced endometrial cancer in whom the administration of pembrolizumab was discontinued after the completion of the 7th course due to renal dysfunction; however, persistent tumor-reducing effects and decreases in the levels of tumor markers were noted for more than 18 months after the cessation of treatment. Pembrolizumab may be continuously administered to some patients for a long period, whereas a durable response is achieved by others even after its discontinuation at an early stage; therefore, difficulties are associated with selecting an appropriate duration of administration. Further studies are required to search for biomarkers that facilitate high-accuracy effect predictions, and to establish an optimal administration period in consideration of specific adverse reactions to ICIs and cost-effectiveness.
Keywords: durable response; endometrial cancer; gynecologic oncology; immune checkpoint inhibitor; pembrolizumab.
Copyright: © Umihira et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
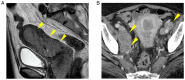

References
-
- Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord JP, Geva R, Gottfried M, Penel N, Hansen AR, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: Results from the phase II KEYNOTE-158 study. J Clin Oncol. 2020;38:1–10. doi: 10.1200/JCO.19.02105. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
