SARS-CoV-2 detection using a nanobody-functionalized voltammetric device
- PMID: 35619829
- PMCID: PMC9126950
- DOI: 10.1038/s43856-022-00113-8
SARS-CoV-2 detection using a nanobody-functionalized voltammetric device
Abstract
Background: An ongoing need during the COVID-19 pandemic has been the requirement for accurate and efficient point-of-care testing platforms to distinguish infected from non-infected people, and to differentiate SARS-CoV-2 infections from other viruses. Electrochemical platforms can detect the virus via its envelope spike protein by recording changes in voltammetric signals between samples. However, this remains challenging due to the limited sensitivity of these sensing platforms.
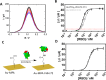
Methods: Here, we report on a nanobody-functionalized electrochemical platform for the rapid detection of whole SARS-CoV-2 viral particles in complex media such as saliva and nasopharyngeal swab samples. The sensor relies on the functionalization of gold electrode surface with highly-oriented Llama nanobodies specific to the spike protein receptor binding domain (RBD). The device provides results in 10 min of exposure to 200 µL of unprocessed samples with high specificity to SARS-CoV-2 viral particles in human saliva and nasopharyngeal swab samples.
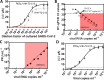
Results: The developed sensor could discriminate between different human coronavirus strains and other respiratory viruses, with 90% positive and 90% negative percentage agreement on 80 clinical samples, as compared to RT-qPCR.
Conclusions: We believe this diagnostic concept, also validated for RBD mutants and successfully tested on Delta variant samples, to be a powerful tool to detect patients' infection status, easily extendable to other viruses and capable of overcoming sensing-related mutation effects.
Keywords: Diagnostic markers; Nanobiotechnology.
© The Author(s) 2022.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.
Figures






References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

