Unexpected Consequences of Noise-Induced Hearing Loss: Impaired Hippocampal Neurogenesis, Memory, and Stress
- PMID: 35619926
- PMCID: PMC9127992
- DOI: 10.3389/fnint.2022.871223
Unexpected Consequences of Noise-Induced Hearing Loss: Impaired Hippocampal Neurogenesis, Memory, and Stress
Abstract
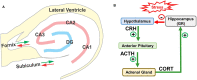
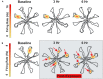
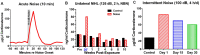
Noise-induced hearing loss (NIHL), caused by direct damage to the cochlea, reduces the flow of auditory information to the central nervous system, depriving higher order structures, such as the hippocampus with vital sensory information needed to carry out complex, higher order functions. Although the hippocampus lies outside the classical auditory pathway, it nevertheless receives acoustic information that influence its activity. Here we review recent results that illustrate how NIHL and other types of cochlear hearing loss disrupt hippocampal function. The hippocampus, which continues to generate new neurons (neurogenesis) in adulthood, plays an important role in spatial navigation, memory, and emotion. The hippocampus, which contains place cells that respond when a subject enters a specific location in the environment, integrates information from multiple sensory systems, including the auditory system, to develop cognitive spatial maps to aid in navigation. Acute exposure to intense noise disrupts the place-specific firing patterns of hippocampal neurons, "spatially disorienting" the cells for days. More traumatic sound exposures that result in permanent NIHL chronically suppresses cell proliferation and neurogenesis in the hippocampus; these structural changes are associated with long-term spatial memory deficits. Hippocampal neurons, which contain numerous glucocorticoid hormone receptors, are part of a complex feedback network connected to the hypothalamic-pituitary (HPA) axis. Chronic exposure to intense intermittent noise results in prolonged stress which can cause a persistent increase in corticosterone, a rodent stress hormone known to suppress neurogenesis. In contrast, a single intense noise exposure sufficient to cause permanent hearing loss produces only a transient increase in corticosterone hormone. Although basal corticosterone levels return to normal after the noise exposure, glucocorticoid receptors (GRs) in the hippocampus remain chronically elevated. Thus, NIHL disrupts negative feedback from the hippocampus to the HPA axis which regulates the release of corticosterone. Preclinical studies suggest that the noise-induced changes in hippocampal place cells, neurogenesis, spatial memory, and glucocorticoid receptors may be ameliorated by therapeutic interventions that reduce oxidative stress and inflammation. These experimental results may provide new insights on why hearing loss is a risk factor for cognitive decline and suggest methods for preventing this decline.
Keywords: glucocorticoid receptor (GCR); hippocampus; memory; neurogenesis; noise-induced hearing loss; spatial navigation; stress.
Copyright © 2022 Manohar, Chen, Ding, Liu, Wang, Chen, Chen and Salvi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

