The Androstenedione Roche Elecsys immunoassay has superior comparability to the LC-MS/MS assay than the Siemens Immulite immunoassay
- PMID: 35620064
- PMCID: PMC9127399
- DOI: 10.1016/j.plabm.2022.e00279
The Androstenedione Roche Elecsys immunoassay has superior comparability to the LC-MS/MS assay than the Siemens Immulite immunoassay
Abstract
Androstenedione (ASD) is a biomarker used in the diagnostic workup of hyperandrogenism, congenital adrenal hyperplasia, premature adrenarche, and polycystic ovary syndrome (PCOS). The Elecsys ASD competitive electrochemiluminescence immunoassay (Roche Diagnostics, Indianapolis, IN) is a new assay recently available in the US.
Objective: This study evaluated the analytical and clinical performance of the Elecsys ASD assay.
Design & methods: We evaluated the linearity/analytical measuring range (AMR), precision, and accuracy of the Elecsys ASD assay on the cobas e601 analyzer. ASD was measured in serum/plasma in the Elecsys ASD, Immulite (Siemens Medical Solutions USA, Inc. Malvern, PA), and LC-MS/MS assays. Reference intervals (RI) were evaluated across genders, menopausal status, and in children. Statistical analysis was performed using EP evaluator and R program.
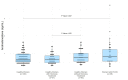
Results: The Elecsys ASD assay had a linear response across the AMR. The intra- and inter-assay coefficients of variation at various concentrations were ≤4.5%. The Elecsys ASD assay had a mean difference of -0.04 ng/mL (-1.7%) with the LC-MS/MS assay, whereas the Immulite assay had a mean difference of 1.17 ng/mL (66%) and -1.22 ng/mL (-38%) compared to the LC-MS/MS and Elecsys ASD assays, respectively. The Roche recommended RIs for healthy men (0.280-1.52 ng/mL) and postmenopausal women (0.187-1.07 ng/mL) were successfully verified. The RIs for children were adopted from published data. For pre-menopausal women, a RI of <1.60 ng/mL was established. The ASD concentrations in women with and without PCOS overlapped.
Conclusions: The Elecsys ASD assay has superior comparability to the LC-MS/MS assay than the Immulite assay.
Keywords: Androstenedione; Immunoassay; LC-MS/MS; Polycystic ovary syndrome; Reference interval.
© 2022 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Jeelani R., Bluth M. In: Williams Textbook of Endocrinology. fourteenth ed. Melmed S., Koenig R., Rosen C., Auchus R., Goldfine A., editors. Elsevier; 2019. Reproductive function and pregnancy.
-
- Nerenz R., Jungheim E., Gronowski A. In: Tietz Textbook of Clinical Chemistry and Molecular Diagnostics. sixth ed. Rifai N., editor. Elsevier; 2018. Reproductive endocrinology and related disorders.
-
- Diagnostics S.H. 2018. Siemens Immulite 2000 Androstenedione.
LinkOut - more resources
Full Text Sources

