Changes in Macrophage Polarization During Tendon-to-Bone Healing After ACL Reconstruction With Insertion-Preserved Hamstring Tendon: Results in a Rabbit Model
- PMID: 35620112
- PMCID: PMC9128061
- DOI: 10.1177/23259671221090894
Changes in Macrophage Polarization During Tendon-to-Bone Healing After ACL Reconstruction With Insertion-Preserved Hamstring Tendon: Results in a Rabbit Model
Abstract
Background: Decreasing the proinflammatory M1 macrophages or shifting the polarization status from M1 to M2 phenotype is thought to be beneficial for tendon-to-bone healing. In anterior cruciate ligament reconstruction (ACLR), using an insertion-preserved hamstring tendon (IP-HT) graft compared with a free hamstring tendon (FHT) graft has been shown to reduce graft necrosis and improve healing. However, the role of macrophage polarization at the tendon-to-bone interface is unclear.
Hypothesis: ACLR using IP-HT graft would facilitate the phenotype shift from M1 to M2 macrophages at the tendon-to-bone interface.
Study design: Controlled laboratory study.
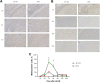
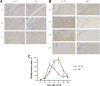
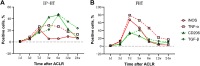
Methods: Unilateral ACLR was performed on 42 healthy New Zealand White rabbits (study group, 21 rabbits with IP-HT graft; control group, 21 rabbits with FHT graft). At days 1, 3, and 7 and weeks 3, 6, 12, and 24 postoperatively, 3 rabbits in each group were sacrificed to investigate and compare the expression of surrogate markers for M1 macrophages (inducible nitric oxide synthase [iNOS] and tumor necrosis factor α [TNF-α]) and M2 macrophages (CD206 and transforming growth factor β [TGF-β]) via immunohistochemical staining and evaluation.
Results: In the control group, the percentage of iNOS- and TNF-α-positive cells from postoperative day 7 and week 3 increased then decreased by week 6; positive expression of CD206 and TGF-β was weaker and peaked at 3 weeks postoperatively. In the study group, high CD206- and TGF-β-positive expression was observed from weeks 3 to 12 and peaked at week 6, and positive expression of iNOS- and TNF-α was weaker and peaked on day 7. At both 7 days and 3 weeks, the percentages of iNOS- and TNF-α-positive cells in the control group were both significantly higher than in the study group (P ≤ .04 for all). At 6 weeks, the percentages of CD206- and TGF-β-positive cells in the study group were both significantly higher than in the control group (P = .02 and P = .04, respectively).
Conclusion: More expression of surrogate markers for M2 macrophages was observed in the tendon-to-bone healing process after ACLR using IP-HT versus FTP graft.
Clinical relevance: Using IP-HT grafts in ACLR may facilitate postoperative healing by shifting the local status of macrophage polarization at the tendon-to-bone interface.
Keywords: ACL; hamstring tendon; macrophage polarization; tendon-to-bone healing.
© The Author(s) 2022.
Conflict of interest statement
This study was funded by National Natural Science Funding (81972062). The authors declared that they have no conflicts of interest in the authorship and publication of this contribution. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Figures


Similar articles
-
Advantages of an Attached Semitendinosus Tendon Graft in Anterior Cruciate Ligament Reconstruction in a Rabbit Model.Am J Sports Med. 2018 Nov;46(13):3227-3236. doi: 10.1177/0363546518799357. Epub 2018 Oct 4. Am J Sports Med. 2018. PMID: 30285459
-
Maturity Progression of the Entire Anterior Cruciate Ligament Graft of Insertion-Preserved Hamstring Tendons by 5 Years: A Prospective Randomized Controlled Study Based on Magnetic Resonance Imaging Evaluation.Am J Sports Med. 2020 Oct;48(12):2970-2977. doi: 10.1177/0363546520951507. Epub 2020 Sep 10. Am J Sports Med. 2020. PMID: 32909826 Clinical Trial.
-
Infrapatellar Fat Pad Mesenchymal Stromal Cell-Derived Exosomes Accelerate Tendon-Bone Healing and Intra-articular Graft Remodeling After Anterior Cruciate Ligament Reconstruction.Am J Sports Med. 2022 Mar;50(3):662-673. doi: 10.1177/03635465211072227. Epub 2022 Feb 28. Am J Sports Med. 2022. PMID: 35224997
-
Anterior Cruciate Ligament Reconstruction: A Systematic Review and Meta-analysis of Outcomes for Quadriceps Tendon Autograft Versus Bone-Patellar Tendon-Bone and Hamstring-Tendon Autografts.Am J Sports Med. 2019 Dec;47(14):3531-3540. doi: 10.1177/0363546518825340. Epub 2019 Feb 21. Am J Sports Med. 2019. PMID: 30790526
-
Tendon Healing in Bone Tunnel after Human Anterior Cruciate Ligament Reconstruction: A Systematic Review of Histological Results.J Knee Surg. 2019 May;32(5):454-462. doi: 10.1055/s-0038-1653964. Epub 2018 May 21. J Knee Surg. 2019. PMID: 29783272
Cited by
-
SHED-derived exosome-mimetics promotes rotator cuff tendon-bone healing via macrophage immunomodulation through NF-κB suppression and autophagy activation.Mater Today Bio. 2025 Jul 28;34:102146. doi: 10.1016/j.mtbio.2025.102146. eCollection 2025 Oct. Mater Today Bio. 2025. PMID: 40809349 Free PMC article.
References
-
- Barrientos S, Stojadinovic OM, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2010;16(5):585–601. - PubMed
-
- Budny J, Fox J, Rauh M, Fineberg M. Emerging trends in anterior cruciate ligament reconstruction. J Knee Surg. 2017;30(1):63–69. - PubMed
-
- Chen W, Sun Y, Gu X, et al. Conditioned medium of human bone marrow-derived stem cells promotes tendon-bone healing of the rotator cuff in a rat model. Biomaterials. 2021;271:120714. - PubMed
LinkOut - more resources
Full Text Sources

