Management Practices During Perinatal Respiratory Transition of Very Premature Infants
- PMID: 35620146
- PMCID: PMC9127974
- DOI: 10.3389/fped.2022.862038
Management Practices During Perinatal Respiratory Transition of Very Premature Infants
Abstract
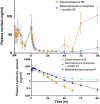
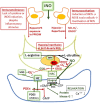
The present review considers some controversial management practices during extremely premature perinatal transition. We focus on perinatal prevention and treatment of respiratory distress syndrome (RDS) in immature infants. New concerns regarding antenatal corticosteroid management have been raised. Many fetuses are only exposed to potential adverse effects of the drug. Hence, the formulation and the dosage may need to be modified. Another challenge is to increase the fraction of the high-risk fetuses that benefit from the drug and to minimize the harmful effects of the drug. On the other hand, boosting anti-inflammatory and anti-microbial properties of surfactant requires further attention. Techniques of prophylactic surfactant administration to extremely immature infants at birth may be further refined. Also, new findings suggest that prophylactic treatment of patent ductus arteriosus (PDA) of a high-risk population rather than later selective closure of PDA may be preferred. The TREOCAPA trial (Prophylactic treatment of the ductus arteriosus in preterm infants by acetaminophen) evaluates, whether early intravenous paracetamol decreases the serious cardiorespiratory consequences following extremely premature birth. Lastly, is inhaled nitric oxide (iNO) used in excess? According to current evidence, iNO treatment of uncomplicated RDS is not indicated. Considerably less than 10% of all very premature infants are affected by early persistence of pulmonary hypertension (PPHN). According to observational studies, effective ventilation combined with early iNO treatment are effective in management of this previously fatal disease. PPHN is associated with prolonged rupture of fetal membranes and birth asphyxia. The lipopolysaccharide (LPS)-induced immunotolerance and hypoxia-reperfusion-induced oxidant stress may inactivate NO-synthetases in pulmonary arterioles and terminal airways. Prospective trials on iNO in the management of PPHN are indicated. Other pulmonary vasodilators may be considered as comparison drugs or adjunctive drugs. The multidisciplinary challenge is to understand the regulation of pregnancy duration and the factors participating the onset of extremely premature preterm deliveries and respiratory adaptation. Basic research aims to identify deficiencies in maternal and fetal tissues that predispose to very preterm births and deteriorate the respiratory adaptation of immature infants. Better understanding on causes and prevention of extremely preterm births would eventually provide effective antenatal and neonatal management practices required for the intact survival.
Keywords: ductus arteriosus; inhaled nitric oxide; paracetamol; persistence of pulmonary hypertension; prenatal steroid; respiratory distress syndrome; spontaneous premature birth; surfactant therapy.
Copyright © 2022 Hallman, Ronkainen, Saarela and Marttila.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Hallman M, Saarela T, Zimmermann LJI. Respiratory distress syndrome: predisposing factors, pathophysiology, and diagnosis. In: Buonocore G, Bracci R, Weindling M. editors, Neonatology. A Practical Approach to Neonatal Diseases. Berlin: Springer; (2018). p. 823–42.
Publication types
LinkOut - more resources
Full Text Sources

