Graph theoretical measures of fast ripples support the epileptic network hypothesis
- PMID: 35620169
- PMCID: PMC9128387
- DOI: 10.1093/braincomms/fcac101
Graph theoretical measures of fast ripples support the epileptic network hypothesis
Abstract
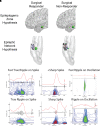
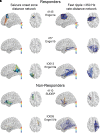
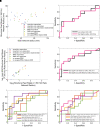
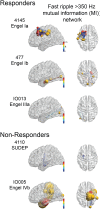
The epileptic network hypothesis and epileptogenic zone hypothesis are two theories of ictogenesis. The network hypothesis posits that coordinated activity among interconnected nodes produces seizures. The epileptogenic zone hypothesis posits that distinct regions are necessary and sufficient for seizure generation. High-frequency oscillations, and particularly fast ripples, are thought to be biomarkers of the epileptogenic zone. We sought to test these theories by comparing high-frequency oscillation rates and networks in surgical responders and non-responders, with no appreciable change in seizure frequency or severity, within a retrospective cohort of 48 patients implanted with stereo-EEG electrodes. We recorded inter-ictal activity during non-rapid eye movement sleep and semi-automatically detected and quantified high-frequency oscillations. Each electrode contact was localized in normalized coordinates. We found that the accuracy of seizure onset zone electrode contact classification using high-frequency oscillation rates was not significantly different in surgical responders and non-responders, suggesting that in non-responders the epileptogenic zone partially encompassed the seizure onset zone(s) (P > 0.05). We also found that in the responders, fast ripple on oscillations exhibited a higher spectral content in the seizure onset zone compared with the non-seizure onset zone (P < 1 × 10-5). By contrast, in the non-responders, fast ripple had a lower spectral content in the seizure onset zone (P < 1 × 10-5). We constructed two different networks of fast ripple with a spectral content >350 Hz. The first was a rate-distance network that multiplied the Euclidian distance between fast ripple-generating contacts by the average rate of fast ripple in the two contacts. The radius of the rate-distance network, which excluded seizure onset zone nodes, discriminated non-responders, including patients not offered resection or responsive neurostimulation due to diffuse multifocal onsets, with an accuracy of 0.77 [95% confidence interval (CI) 0.56-0.98]. The second fast ripple network was constructed using the mutual information between the timing of the events to measure functional connectivity. For most non-responders, this network had a longer characteristic path length, lower mean local efficiency in the non-seizure onset zone, and a higher nodal strength among non-seizure onset zone nodes relative to seizure onset zone nodes. The graphical theoretical measures from the rate-distance and mutual information networks of 22 non- responsive neurostimulation treated patients was used to train a support vector machine, which when tested on 13 distinct patients classified non-responders with an accuracy of 0.92 (95% CI 0.75-1). These results indicate patients who do not respond to surgery or those not selected for resection or responsive neurostimulation can be explained by the epileptic network hypothesis that is a decentralized network consisting of widely distributed, hyperexcitable fast ripple-generating nodes.
Keywords: brain network; epilepsy surgery; fast ripple; high-frequency oscillation.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures




References
-
- Rosenow F, Lüders H. Presurgical evaluation of epilepsy. Brain. 2001;124(9):1683–1700. - PubMed
-
- Lüders HO, Najm I, Nair D, Widdess-Walsh P, Bingman W. The epileptogenic zone: General principles. Epileptic Disord. 2006;8(Suppl 2):S1–S9. - PubMed
-
- Spencer SS. Neural networks in human epilepsy: Evidence of and implications for treatment. Epilepsia. 2002;43(3):219–227. - PubMed
-
- Richardson MP. Large scale brain models of epilepsy: Dynamics meets connectomics. J Neurol Neurosurg Psychiatry. 2012;83(12):1238–1248. - PubMed
