Mechanism of Fructus Mume Pills Underlying Their Protective Effects in Rats with Acetic Acid-Inducedulcerative Colitis via the Regulation of Inflammatory Cytokines and the VEGF-PI3K/Akt-eNOS Signaling Pathway
- PMID: 35620404
- PMCID: PMC9129976
- DOI: 10.1155/2022/4621131
Mechanism of Fructus Mume Pills Underlying Their Protective Effects in Rats with Acetic Acid-Inducedulcerative Colitis via the Regulation of Inflammatory Cytokines and the VEGF-PI3K/Akt-eNOS Signaling Pathway
Abstract
Background: Fructus mume pills (FMPs) have been clinically proven to be effective for treating ulcerative colitis (UC). However, the therapeutic and protective mechanisms have not been fully studied.
Aim: We aimed to explore the mechanism of FMPs in an acetic acid (AA)-induced ulcerative colitis rat model.
Methods: The targets, GO terms, and KEGG pathways for the FMPs and UC were screened and constructed using network pharmacology. A possible mechanism was verified in a 4% AA-induced colitis rat model. Colitis activity and state were evaluated using the disease activity index, and colon ulceration and intestinal mucosal damage were determined by histopathological observation through HE, AB-PAS, and Masson pathological staining. The concentrations of TNF-α, IL-6, IL-8, IL-10, MPO, MMP9, CXCR1, eNOS, and VEGF were measured to evaluate vascular permeability effects.
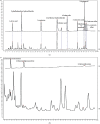
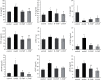
Results: The network pharmacology results showed 108 active compounds, and 139 FMP-related targets were identified. Twenty-nine targets were identified for FMPs against UC, which included MMP9, MMP3, ESR1, PTGS1, PPARA, MPO, and NOS2. A total of 1,536 GO terms and 41 pathways were associated with FMP treatment of UC. The pharmacological evaluation showed that FMPs attenuated inflammation in AA-induced colitis by reducing the serum concentrations of TNF-α, IL-6, IL-8, and IL-10 and the colonic concentrations of MPO, MMP9, and CXCR1. FMPs ameliorated hyperpermeability by reducing the colonic VEGF and eNOS concentrations. FMPs also significantly decreased the VEGFA, VEGFR2, Src, and eNOS protein expressions in colon tissue through the VEGF-PI3K/Akt-eNOS signaling pathway.
Conclusion: These results suggest that FMPs control UC inflammation by regulating inflammatory cytokine concentrations. FMPs alleviate AA-induced UC by regulating microvascular permeability through the VEGF-PI3K/Akt-eNOS signaling pathway.
Copyright © 2022 Zongying Xu et al.
Conflict of interest statement
The authors declare no competing interests.
Figures







Similar articles
-
Fructus Mume (Wu Mei) Attenuates Acetic Acid-Induced Ulcerative Colitis by Regulating Inflammatory Cytokine, Reactive Oxygen Species, and Neuropeptide Levels in Model Rats.J Med Food. 2022 Apr;25(4):389-401. doi: 10.1089/jmf.2021.K.0155. J Med Food. 2022. PMID: 35438553
-
Exploring the mechanism of the Fructus Mume and Rhizoma Coptidis herb pair intervention in Ulcerative Colitis from the perspective of inflammation and immunity based on systemic pharmacology.BMC Complement Med Ther. 2023 Jan 16;23(1):11. doi: 10.1186/s12906-022-03823-7. BMC Complement Med Ther. 2023. PMID: 36647064 Free PMC article.
-
Renshen Baidu Powder Attenuated Intestinal Inflammation and Apoptosis in Ulcerative Colitis Rats through the Inhibition of PI3K/AKT/NF-κB Signaling Pathway.Evid Based Complement Alternat Med. 2022 Jul 30;2022:5234025. doi: 10.1155/2022/5234025. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 35942369 Free PMC article.
-
Study on the mechanism of Huangqin Decoction on rats with ulcerative colitis of damp-heat type base on mtDNA, TLR4, p-PI3K, p-Akt protein expression and microbiota.J Ethnopharmacol. 2022 Sep 15;295:115356. doi: 10.1016/j.jep.2022.115356. Epub 2022 May 11. J Ethnopharmacol. 2022. PMID: 35568112
-
Aloe vera mitigates dextran sulfate sodium-induced rat ulcerative colitis by potentiating colon mucus barrier.J Ethnopharmacol. 2021 Oct 28;279:114108. doi: 10.1016/j.jep.2021.114108. Epub 2021 Apr 8. J Ethnopharmacol. 2021. PMID: 33839199
Cited by
-
Physiologically-Modeled Dynamic Stimulation and Growth Factors Induce Differentiation of Mesenchymal Stem Cells to a Vascular Endothelial Cell Phenotype.Microcirculation. 2025 Apr;32(3):e70007. doi: 10.1111/micc.70007. Microcirculation. 2025. PMID: 40120632
-
Effect of Dietary Fructus mume and Scutellaria baicalensis Georgi on the Fecal Microbiota and Its Correlation with Apparent Nutrient Digestibility in Weaned Piglets.Animals (Basel). 2022 Sep 14;12(18):2418. doi: 10.3390/ani12182418. Animals (Basel). 2022. PMID: 36139277 Free PMC article.
-
Protective effect of Dulaglutide, a GLP1 agonist, on acetic acid-induced ulcerative colitis in rats: involvement of GLP-1, TFF-3, and TGF-β/PI3K/NF-κB signaling pathway.Naunyn Schmiedebergs Arch Pharmacol. 2025 May;398(5):5611-5628. doi: 10.1007/s00210-024-03631-5. Epub 2024 Nov 23. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 39579211 Free PMC article.
-
Evaluation of the Mechanism of Sinomenii Caulis in Treating Ulcerative Colitis based on Network Pharmacology and Molecular Docking.Curr Comput Aided Drug Des. 2024;20(3):195-207. doi: 10.2174/1573409919666230420083102. Curr Comput Aided Drug Des. 2024. PMID: 37078344 Free PMC article.
-
Protective Application of Chinese Herbal Compounds and Formulae in Intestinal Inflammation in Humans and Animals.Molecules. 2023 Sep 26;28(19):6811. doi: 10.3390/molecules28196811. Molecules. 2023. PMID: 37836654 Free PMC article. Review.
References
-
- Zhang S. S., Shen H., Zhen K., et al. Ulcerative colitis diagnosis and treatment experts consensus(2017) Chinese journal of traditional Chinese medicine . 2017;32(08):3585–3589. (in Chinese)
-
- van der Valk M. E., Mangen M.-J. J., Severs M., et al. Comparison of costs and quality of life in ulcerative colitis patients with an ileal pouch-anal anastomosis, ileostomy and anti-tnfα therapy. Journal of Crohn’s and Colitis . 2015 Nov;9(11):1016–1023. doi: 10.1093/ecco-jcc/jjv134. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

