Critical Implications of IVDR for Innovation in Diagnostics: Input From the BioMed Alliance Diagnostics Task Force
- PMID: 35620593
- PMCID: PMC9126521
- DOI: 10.1097/HS9.0000000000000724
Critical Implications of IVDR for Innovation in Diagnostics: Input From the BioMed Alliance Diagnostics Task Force
Figures

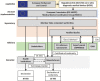

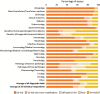
References
-
- Directive 98/79/EC of the European Parliament and of the Council of 27 October 1998 on in vitro diagnostic medical devices. Official J Eur Commun. 1998;331:1–37.
-
- Regulation (EU) 2017/746 of the European Parliament and of the Council of 5 April 2017 on in vitro diagnostic medical devices and repealing Directive 98/79/EC and Commission Decision 2010/227/EU. Official J Eur Union. 2017;117:176–332.
-
- Lubbers BR, van Dongen JJM. Position of European Consortia in the IVDR Era: Support for In-house Devices (IH-IVDs) and CE Marked IVDs (CE-IVDs). 9th ESLHO Symposium Abstract Book. 2020:33–46. Available at: https://eslho-public.s3.nl-ams.scw.cloud/Lubbers2020_94364d9779.pdf. Accessed March 5, 2022.
-
- Medical Device Coordination Group. MDCG 2022-2 Guidance on General Principles of Clinical Evidence for In Vitro Diagnostic Medical Devices (IVDs). 2022. Available at: https://ec.europa.eu/health/system/files/2022-01/mdcg_2022-2_en.pdf. Accessed March 5, 2022.
LinkOut - more resources
Full Text Sources
