Combined Drought and Heat Stress Influences the Root Water Relation and Determine the Dry Root Rot Disease Development Under Field Conditions: A Study Using Contrasting Chickpea Genotypes
- PMID: 35620681
- PMCID: PMC9128860
- DOI: 10.3389/fpls.2022.890551
Combined Drought and Heat Stress Influences the Root Water Relation and Determine the Dry Root Rot Disease Development Under Field Conditions: A Study Using Contrasting Chickpea Genotypes
Abstract
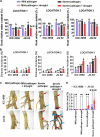
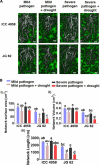
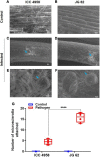
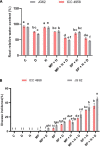
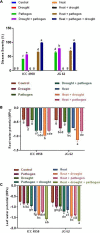
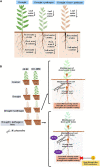
Abiotic stressors such as drought and heat predispose chickpea plants to pathogens of key importance leading to significant crop loss under field conditions. In this study, we have investigated the influence of drought and high temperature on the incidence and severity of dry root rot disease (caused by Macrophomina phaseolina) in chickpea, under extensive on- and off-season field trials and greenhouse conditions. We explored the association between drought tolerance and dry root rot resistance in two chickpea genotypes, ICC 4958 and JG 62, with contrasting resistance to dry root rot. In addition, we extensively analyzed various patho-morphological and root architecture traits altered by combined stresses under field and greenhouse conditions in these genotypes. We further observed the role of edaphic factors in dry root rot incidence under field conditions. Altogether, our results suggest a strong negative correlation between the plant water relations and dry root rot severity in chickpeas, indicating an association between drought tolerance and dry root rot resistance. Additionally, the significant role of heat stress in altering the dynamics of dry root rot and the importance of combinatorial screening of chickpea germplasm for dry root rot resistance, drought, and heat stress have been revealed.
Keywords: Cicer arietinum; Macrophomina phaseolina; combined stress; disease resistance; drought; heat; plant water status; stress tolerance.
Copyright © 2022 Chilakala, Mali, Irulappan, Patil, Pandey, Rangappa, Ramegowda, Kumar, Puli, Mohan-Raju and Senthil-Kumar.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Cabral R. N., Marouelli W. A., Café-Filho A. C. (2020). Irrigation management strategies for reducing verticillium wilt severity in eggplants. Summa Phytopathol. 46 9–13. 10.1590/0100-5405/190000 - DOI
-
- de Sousa Linhares C. M., Ambrósio M. M. Q., Castro G., Torres S. B., Esteras C., De Sousa Nunes G. H., et al. (2020). Effect of temperature on disease severity of charcoal rot of melons caused by Macrophomina phaseolina: implications for selection of resistance sources. Eur. J. Plant Pathol. 158 431–441. 10.1007/s10658-020-02083-w - DOI
-
- Deokar A. A., Kondawar V., Jain P. K., Karuppayil S. M., Raju N., Vadez V., et al. (2011). Comparative analysis of expressed sequence tags (ESTs) between drought-tolerant and-susceptible genotypes of chickpea under terminal drought stress. BMC Plant Biol. 11:70. 10.1186/1471-2229-11-70 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

