Effectiveness of complete primary vaccination against COVID-19 at primary care and community level during predominant Delta circulation in Europe: multicentre analysis, I-MOVE-COVID-19 and ECDC networks, July to August 2021
- PMID: 35620997
- PMCID: PMC9137272
- DOI: 10.2807/1560-7917.ES.2022.27.21.2101104
Effectiveness of complete primary vaccination against COVID-19 at primary care and community level during predominant Delta circulation in Europe: multicentre analysis, I-MOVE-COVID-19 and ECDC networks, July to August 2021
Abstract
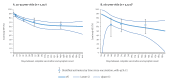
IntroductionIn July and August 2021, the SARS-CoV-2 Delta variant dominated in Europe.AimUsing a multicentre test-negative study, we measured COVID-19 vaccine effectiveness (VE) against symptomatic infection.MethodsIndividuals with COVID-19 or acute respiratory symptoms at primary care/community level in 10 European countries were tested for SARS-CoV-2. We measured complete primary course overall VE by vaccine brand and by time since vaccination.ResultsOverall VE was 74% (95% CI: 69-79), 76% (95% CI: 71-80), 63% (95% CI: 48-75) and 63% (95% CI: 16-83) among those aged 30-44, 45-59, 60-74 and ≥ 75 years, respectively. VE among those aged 30-59 years was 78% (95% CI: 75-81), 66% (95% CI: 58-73), 91% (95% CI: 87-94) and 52% (95% CI: 40-61), for Comirnaty, Vaxzevria, Spikevax and COVID-19 Vaccine Janssen, respectively. VE among people 60 years and older was 67% (95% CI: 52-77), 65% (95% CI: 48-76) and 83% (95% CI: 64-92) for Comirnaty, Vaxzevria and Spikevax, respectively. Comirnaty VE among those aged 30-59 years was 87% (95% CI: 83-89) at 14-29 days and 65% (95% CI: 56-71%) at ≥ 90 days between vaccination and onset of symptoms.ConclusionsVE against symptomatic infection with the SARS-CoV-2 Delta variant varied among brands, ranging from 52% to 91%. While some waning of the vaccine effect may be present (sample size limited this analysis to only Comirnaty), protection was 65% at 90 days or more between vaccination and onset.
Keywords: COVID-19; Delta variant; Europe; SARS-CoV-2; multicentre study; test-negative design; vaccine effectiveness.
Conflict of interest statement
Figures

References
-
- World Health Organisation (WHO). COVID-19 Weekly Epidemiological Update. Edition 56. Geneva: WHO; 2021. Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/2...
-
- European Centre for Disease Prevention and Control (ECDC), World Health Organization Regional Office for Europe (WHO/Europe). Assessing SARS-CoV-2 circulation, variants of concern, non-pharmaceutical interventions and vaccine rollout in the EU/EEA, 16th update. Stockholm: ECDC; 2021. Available from: https://www.ecdc.europa.eu/sites/default/files/documents/covid-19-rapid-...
-
- Nextstrain. Genomic epidemiology of SARS-CoV-2 with Europe-focused subsampling. [Accessed: 30 Oct 2021]. Available from: https://nextstrain.org/ncov/gisaid/europe
-
- European Medicines Agency (EMA). COVID-19 vaccines: authorised. Amsterdam: EMA. [Accessed: 28 Apr 2022]. Available from: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-thr... vaccines
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
