CRISPR/Cas9 and genetic screens in malaria parasites: small genomes, big impact
- PMID: 35621119
- PMCID: PMC9246331
- DOI: 10.1042/BST20210281
CRISPR/Cas9 and genetic screens in malaria parasites: small genomes, big impact
Abstract
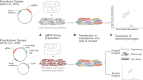
The ∼30 Mb genomes of the Plasmodium parasites that cause malaria each encode ∼5000 genes, but the functions of the majority remain unknown. This is due to a paucity of functional annotation from sequence homology, which is compounded by low genetic tractability compared with many model organisms. In recent years technical breakthroughs have made forward and reverse genome-scale screens in Plasmodium possible. Furthermore, the adaptation of Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) and CRISPR-Associated protein 9 (CRISPR/Cas9) technology has dramatically improved gene editing efficiency at the single gene level. Here, we review the arrival of genetic screens in malaria parasites to analyse parasite gene function at a genome-scale and their impact on understanding parasite biology. CRISPR/Cas9 screens, which have revolutionised human and model organism research, have not yet been implemented in malaria parasites due to the need for more complex CRISPR/Cas9 gene targeting vector libraries. We therefore introduce the reader to CRISPR-based screens in the related apicomplexan Toxoplasma gondii and discuss how these approaches could be adapted to develop CRISPR/Cas9 based genome-scale genetic screens in malaria parasites. Moreover, since more than half of Plasmodium genes are required for normal asexual blood-stage reproduction, and cannot be targeted using knockout methods, we discuss how CRISPR/Cas9 could be used to scale up conditional gene knockdown approaches to systematically assign function to essential genes.
Keywords: Plasmodium falciparum; CRISPR; biochemical techniques and resources; genetics; malaria.
© 2022 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures


Similar articles
-
Optimizing Systems for Cas9 Expression in Toxoplasma gondii.mSphere. 2019 Jun 26;4(3):e00386-19. doi: 10.1128/mSphere.00386-19. mSphere. 2019. PMID: 31243081 Free PMC article.
-
CRISPR/Cas9 Gene Editing to Make Conditional Mutants of Human Malaria Parasite P. falciparum.J Vis Exp. 2018 Sep 18;(139):57747. doi: 10.3791/57747. J Vis Exp. 2018. PMID: 30295650 Free PMC article.
-
Cutting back malaria: CRISPR/Cas9 genome editing of Plasmodium.Brief Funct Genomics. 2019 Sep 24;18(5):281-289. doi: 10.1093/bfgp/elz012. Brief Funct Genomics. 2019. PMID: 31365053 Free PMC article. Review.
-
Application of the CRISPR/Cas9 gene editing technique to research on functional genomes of parasites.Parasitol Int. 2016 Dec;65(6 Pt A):641-644. doi: 10.1016/j.parint.2016.08.011. Epub 2016 Aug 30. Parasitol Int. 2016. PMID: 27586395 Review.
-
Development of CRISPR/Cas9 for Efficient Genome Editing in Toxoplasma gondii.Methods Mol Biol. 2017;1498:79-103. doi: 10.1007/978-1-4939-6472-7_6. Methods Mol Biol. 2017. PMID: 27709570
Cited by
-
Comparative chemical genomics in Babesia species identifies the alkaline phosphatase PhoD as a determinant of antiparasitic resistance.Proc Natl Acad Sci U S A. 2024 Feb 27;121(9):e2312987121. doi: 10.1073/pnas.2312987121. Epub 2024 Feb 20. Proc Natl Acad Sci U S A. 2024. PMID: 38377214 Free PMC article.
-
A scaleable inducible knockout system for studying essential gene function in the malaria parasite.Nucleic Acids Res. 2025 Feb 8;53(4):gkae1274. doi: 10.1093/nar/gkae1274. Nucleic Acids Res. 2025. PMID: 39739757 Free PMC article.
-
The dCas9-based genome editing in Plasmodium yoelii.mSphere. 2024 Mar 26;9(3):e0009524. doi: 10.1128/msphere.00095-24. Epub 2024 Feb 27. mSphere. 2024. PMID: 38411120 Free PMC article.
-
Plasmodium yoelii surface-related antigen (PySRA) modulates the host pro-inflammatory responses via binding to CD68 on macrophage membrane.Infect Immun. 2024 May 7;92(5):e0011324. doi: 10.1128/iai.00113-24. Epub 2024 Apr 16. Infect Immun. 2024. PMID: 38624215 Free PMC article.
-
A scalable CRISPR-Cas9 gene editing system facilitates CRISPR screens in the malaria parasite Plasmodium berghei.Nucleic Acids Res. 2025 Jan 11;53(2):gkaf005. doi: 10.1093/nar/gkaf005. Nucleic Acids Res. 2025. PMID: 39844455 Free PMC article.
References
-
- World malaria report 2021. (2021) World Health Organization, Geneva. Available from: https://www.who.int/publications/i/item/9789240040496
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

