Mechanical Bond Enhanced Lithium Halide Ion-Pair Binding by Halogen Bonding Heteroditopic Rotaxanes
- PMID: 35621330
- PMCID: PMC9541756
- DOI: 10.1002/chem.202201209
Mechanical Bond Enhanced Lithium Halide Ion-Pair Binding by Halogen Bonding Heteroditopic Rotaxanes
Abstract
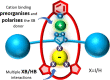
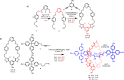
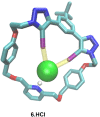
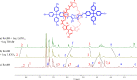
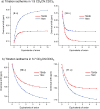
A family of novel halogen bonding (XB) and hydrogen bonding (HB) heteroditopic [2]rotaxane host systems constructed by active metal template (AMT) methodology, were studied for their ability to cooperatively recognise lithium halide (LiX) ion-pairs. 1 H NMR ion-pair titration experiments in CD3 CN:CDCl3 solvent mixtures revealed a notable "switch-on" of halide anion binding in the presence of a co-bound lithium cation, with rotaxane hosts demonstrating selectivity for LiBr over LiI. The strength of halide binding was shown to greatly increase with increasing number of halogen bond donors integrated into the interlocked cavity, where an all-XB rotaxane was found to be the most potent host for LiBr. DFT calculations corroborated these findings, determining the mode of LiX ion-pair binding. Notably, ion-pair binding was not observed with the corresponding XB/HB macrocycles alone, highlighting the cooperative, heteroditopic, rotaxane axle-macrocycle component mechanical bond effect as an efficient strategy for ion-pair recognition in general.
Keywords: Ion-pair; halogen bond; heteroditopic rotaxane; hydrogen bond; lithium halide.
© 2022 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
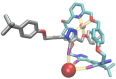
References
-
- Arduini A., Casnati A., Pochini A., Ungaro R., Curr. Opin. Chem. Biol. 1997, 1, 467–474. - PubMed
-
- Busschaert N., Caltagirone C., Van Rossom W., Gale P. A., Chem. Rev. 2015, 115, 8038–8155. - PubMed
-
- Beer P. D., Gale P. A., Angew. Chem. Int. Ed. 2001, 40, 486–516; - PubMed
- Angew. Chem. 2001, 113, 502–532.
-
- Evans N. H., Beer P. D., Angew. Chem. Int. Ed. 2014, 53, 11716–11754; - PubMed
- Angew. Chem. 2014, 126, 11908–11948.
LinkOut - more resources
Full Text Sources
Miscellaneous

