Gold Nanorod-Assisted Photothermal Therapy and Improvement Strategies
- PMID: 35621478
- PMCID: PMC9138169
- DOI: 10.3390/bioengineering9050200
Gold Nanorod-Assisted Photothermal Therapy and Improvement Strategies
Abstract
Noble metal nanoparticles have been sought after in cancer nanomedicine during the past two decades, owing to the unique localized surface plasmon resonance that induces strong absorption and scattering properties of the nanoparticles. A popular application of noble metal nanoparticles is photothermal therapy, which destroys cancer cells by heat generated by laser irradiation of the nanoparticles. Gold nanorods have stood out as one of the major types of noble metal nanoparticles for photothermal therapy due to the facile tuning of their optical properties in the tissue penetrative near infrared region, strong photothermal conversion efficiency, and long blood circulation half-life after surface modification with stealthy polymers. In this review, we will summarize the optical properties of gold nanorods and their applications in photothermal therapy. We will also discuss the recent strategies to improve gold nanorod-assisted photothermal therapy through combination with chemotherapy and photodynamic therapy.
Keywords: cancer; gold nanorod; photothermal therapy; surface plasmon resonance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

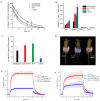


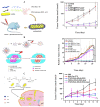

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

