Human-Origin iPSC-Based Recellularization of Decellularized Whole Rat Livers
- PMID: 35621497
- PMCID: PMC9137624
- DOI: 10.3390/bioengineering9050219
Human-Origin iPSC-Based Recellularization of Decellularized Whole Rat Livers
Abstract
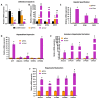
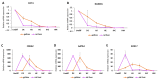
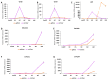
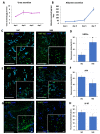
End-stage liver diseases lead to mortality of millions of patients, as the only treatment available is liver transplantation and donor scarcity means that patients have to wait long periods before receiving a new liver. In order to minimize donor organ scarcity, a promising bioengineering approach is to decellularize livers that do not qualify for transplantation. Through decellularization, these organs can be used as scaffolds for developing new functional organs. In this process, the original cells of the organ are removed and ideally should be replaced by patient-specific cells to eliminate the risk of immune rejection. Induced pluripotent stem cells (iPSCs) are ideal candidates for developing patient-specific organs, yet the maturity and functionality of iPSC-derived cells do not match those of primary cells. In this study, we introduced iPSCs into decellularized rat liver scaffolds prior to the start of differentiation into hepatic lineages to maximize the exposure of iPSCs to native liver matrices. Through exposure to the unique composition and native 3D organization of the liver microenvironment, as well as the more efficient perfusion culture throughout the differentiation process, iPSC differentiation into hepatocyte-like cells was enhanced. The resulting cells showed significantly higher expression of mature hepatocyte markers, including important CYP450 enzymes, along with lower expression of fetal markers, such as AFP. Importantly, the gene expression profile throughout the different stages of differentiation was more similar to native development. Our study shows that the native 3D liver microenvironment has a pivotal role to play in the development of human-origin hepatocyte-like cells with more mature characteristics.
Keywords: decellularization; iPSCs; liver bioengineering; recellularization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- United Network for Organ Sharing (UNOS) Transplant Trends. [(accessed on 4 May 2020)]. Available online: https://unos.org/data/transplant-trends/
-
- Sabetkish S., Kajbafzadeh A.-M., Sabetkish N., Khorramirouz R., Akbarzadeh A., Seyedian S.L., Pasalar P., Orangian S., Beigi R.S.H., Aryan Z., et al. Whole-organ tissue engineering: Decellularization and recellularization of three-dimensional matrix liver scaffolds. J. Biomed. Mater. Res. A. 2015;103:1498–1508. doi: 10.1002/jbm.a.35291. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

