Immune Checkpoint Inhibitors in Cancer Therapy
- PMID: 35621637
- PMCID: PMC9139602
- DOI: 10.3390/curroncol29050247
Immune Checkpoint Inhibitors in Cancer Therapy
Abstract
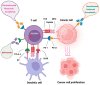
The discovery of immune checkpoint proteins such as PD-1/PDL-1 and CTLA-4 represents a significant breakthrough in the field of cancer immunotherapy. Therefore, humanized monoclonal antibodies, targeting these immune checkpoint proteins have been utilized successfully in patients with metastatic melanoma, renal cell carcinoma, head and neck cancers and non-small lung cancer. The US FDA has successfully approved three different categories of immune checkpoint inhibitors (ICIs) such as PD-1 inhibitors (Nivolumab, Pembrolizumab, and Cemiplimab), PDL-1 inhibitors (Atezolimumab, Durvalumab and Avelumab), and CTLA-4 inhibitor (Ipilimumab). Unfortunately, not all patients respond favourably to these drugs, highlighting the role of biomarkers such as Tumour mutation burden (TMB), PDL-1 expression, microbiome, hypoxia, interferon-γ, and ECM in predicting responses to ICIs-based immunotherapy. The current study aims to review the literature and updates on ICIs in cancer therapy.
Keywords: CTLA-4; PD-1; PD-L1; immune checkpoint inhibitors; immunotherapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials