Identification and Characterization of Chemosensory Receptors in the Pheromone Gland-Ovipositor of Spodoptera frugiperda (J. E. Smith)
- PMID: 35621815
- PMCID: PMC9146910
- DOI: 10.3390/insects13050481
Identification and Characterization of Chemosensory Receptors in the Pheromone Gland-Ovipositor of Spodoptera frugiperda (J. E. Smith)
Abstract
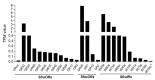
Chemoreception by moth ovipositors has long been suggested, but underlying molecular mechanisms are mostly unknown. To reveal such chemosensory systems in the current study, we sequenced and assembled the pheromone gland-ovipositor (PG-OV) transcriptome of females of the fall armyworm, Spodoptera frugiperda, a pest of many crops. We annotated a total of 26 candidate chemosensory receptor genes, including 12 odorant receptors (ORs), 4 gustatory receptors (GRs), and 10 ionotropic receptors (IRs). The relatedness of these chemosensory receptors with those from other insect species was predicted by phylogenetic analyses, and specific genes, including pheromone receptors, ORco, CO2 receptors, sugar receptors, and IR co-receptors, were reported. Although real-time quantitative-PCR analyses of annotated genes revealed that OR and IR genes were mainly expressed in S. frugiperda antennae, two ORs and two IRs expressed in antennae were also highly expressed in the PG-OV. Similarly, GR genes were mainly expressed in the proboscis, but two were also highly expressed in the PG-OV. Our study provides the first large-scale description of chemosensory receptors in the PG-OV of S. frugiperda and provides a foundation for exploring the chemoreception mechanisms of PG-OV in S. frugiperda and in other moth species.
Keywords: Spodoptera frugiperda; gustatory receptor; ionotropic receptor; odorant receptor; pheromone gland-ovipositor; real-time quantitative PCR; transcriptome.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


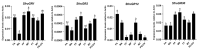

Similar articles
-
Candidate chemosensory receptors in the antennae and maxillae of Spodoptera frugiperda (J. E. Smith) larvae.Front Physiol. 2022 Sep 15;13:970915. doi: 10.3389/fphys.2022.970915. eCollection 2022. Front Physiol. 2022. PMID: 36187799 Free PMC article.
-
A mouthpart transcriptome for Spodoptera frugiperda adults: identification of candidate chemoreceptors and investigation of expression patterns.Front Physiol. 2023 Apr 25;14:1193085. doi: 10.3389/fphys.2023.1193085. eCollection 2023. Front Physiol. 2023. PMID: 37179830 Free PMC article.
-
Identification and expression analysis of chemosensory receptors in the tarsi of fall armyworm, Spodoptera frugiperda (J. E. Smith).Front Physiol. 2023 Apr 10;14:1177297. doi: 10.3389/fphys.2023.1177297. eCollection 2023. Front Physiol. 2023. PMID: 37101698 Free PMC article.
-
Identification of Candidate Chemosensory Receptors in the Antennae of the Variegated Cutworm, Peridroma saucia Hübner, Based on a Transcriptome Analysis.Front Physiol. 2020 Jan 31;11:39. doi: 10.3389/fphys.2020.00039. eCollection 2020. Front Physiol. 2020. PMID: 32082194 Free PMC article.
-
Identification and characterization of chemosensory genes in the antennal transcriptome of Spodoptera exigua.Comp Biochem Physiol Part D Genomics Proteomics. 2018 Sep;27:54-65. doi: 10.1016/j.cbd.2018.05.001. Epub 2018 May 7. Comp Biochem Physiol Part D Genomics Proteomics. 2018. PMID: 29787920
Cited by
-
Odorant receptors for floral- and plant-derived volatiles in the yellow fever mosquito, Aedes aegypti (Diptera: Culicidae).PLoS One. 2024 May 6;19(5):e0302496. doi: 10.1371/journal.pone.0302496. eCollection 2024. PLoS One. 2024. PMID: 38709760 Free PMC article.
-
Molecule characterization of chemosensory and metabolism-related genes in the proboscis of Athetis lepigone.Front Physiol. 2023 Dec 22;14:1287353. doi: 10.3389/fphys.2023.1287353. eCollection 2023. Front Physiol. 2023. PMID: 38187138 Free PMC article.
-
Chemosensory Receptor Expression in the Abdomen Tip of the Female Codling Moth, Cydia pomonella L. (Lepidoptera: Tortricidae).Insects. 2023 Dec 14;14(12):948. doi: 10.3390/insects14120948. Insects. 2023. PMID: 38132621 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

