Blood Pressure Increase following COVID-19 Vaccination: A Systematic Overview and Meta-Analysis
- PMID: 35621861
- PMCID: PMC9147472
- DOI: 10.3390/jcdd9050150
Blood Pressure Increase following COVID-19 Vaccination: A Systematic Overview and Meta-Analysis
Abstract
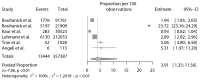
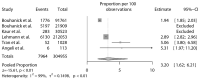
Coronavirus disease 2019 (COVID-19) vaccines proved a strong clinical efficacy against symptomatic or moderate/severe COVID-19 and are considered the most promising approach for curbing the pandemic. However, some questions regarding the safety of COVID-19 vaccines have been recently raised. Among adverse events to vaccines and despite a lack of signal during phase III clinical trials, an increase in blood pressure (BP) after COVID-19 vaccination has been reported as a potential adverse reaction. We systematically analyze this topic and undertook a meta-analysis of available data to estimate the proportion of patients with abnormal BP or raise in BP after vaccination. Six studies entered the final analysis. Overall, studies accrued 357,387 subjects with 13,444 events of abnormal or increased BP. After exclusion of outlier studies, the pooled estimated proportion of abnormal/increased BP after vaccination was 3.20% (95% CI: 1.62-6.21). Proportions of cases of stage III hypertension or hypertensive urgencies and emergencies was 0.6% (95% CI: 0.1% to 5.1%). In conclusion, abnormal BP is not rare after COVID-19 vaccination, but the basic mechanisms of this phenomenon are still unclear and require further research.
Keywords: Ad26.COV2.S; BNT162b2; COVID-19; CVnCoV; ChAdOx1nCoV-19; Gam-COVID-Vac; NVX-CoV2373; adverse drug reaction; blood pressure; hypertension; mRNA-1273; vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Angeli F., Verdecchia P., Balestrino A., Bruschi C., Ceriana P., Chiovato L., Dalla Vecchia L.A., Fanfulla F., La Rovere M.T., Perego F., et al. Renin Angiotensin System Blockers and Risk of Mortality in Hypertensive Patients Hospitalized for COVID-19: An Italian Registry. J. Cardiovasc. Dev. Dis. 2022;9:15. doi: 10.3390/jcdd9010015. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources