Secondary Metabolites Isolated from Chilean Marine Algae: A Review
- PMID: 35621988
- PMCID: PMC9147571
- DOI: 10.3390/md20050337
Secondary Metabolites Isolated from Chilean Marine Algae: A Review
Abstract
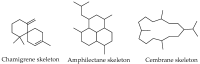
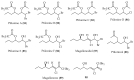
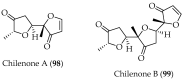
Chile is in the extreme southwestern part of America, and it has an extreme length, of approximately 4300 km that increases to 8000 km considering the Chilean Antarctic Territory. Despite the large extent of its coastal territory and the diversity of geographic environments and climates associated with Chilean coasts, the research on marine resources in Chile has been rather scarce. From marine organisms found in Chilean coastal waters, algae have been the most studied, since they contain a wide range of interesting secondary metabolites that have some structural traits that make them unique and uncharacteristic. Thus, a wide structural variety of natural products including terpenoids (monoterpenes, sesquiterpenes, diterpenes, and meroterpenoids), furanones, and C15-acetogenins have been isolated and identified. This review describes the existing literature on bioprospecting and exploration of secondary metabolites from Chilean coasts.
Keywords: Chilean algae; biological activities; biosynthesis; marine natural products; secondary metabolites; structure elucidation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






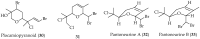










References
-
- San-Martín A., Rovirosa J., Bacho M., Gaete K., Ampuero J. A New Labdane Diterpene from the Limpet Trimusculus Peruvianus. Bol. Latinoam. Caribe Plantas. 2012;11:520.
-
- Mayer A.M.S., Rodríguez A.D., Berlinck R.G.S., Fusetani N. Marine Pharmacology in 2007–2008: Marine Compounds with Antibacterial, Anticoagulant, Antifungal, Anti-Inflammatory, Antimalarial, Antiprotozoal, Antituberculosis, and Antiviral Activities; Affecting the Immune and Nervous System, and Other Miscellaneous Mechanisms of Action. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2011;153:191. - PMC - PubMed
-
- Mayer A., Rodríguez A., Taglialatela-Scafati O., Fusetani N. Marine Pharmacology in 2012–2013: Marine Compounds with Antibacterial, Antidiabetic, Antifungal, Anti-Inflammatory, Antiprotozoal, Antituberculosis, and Antiviral Activities; Affecting the Immune and Nervous Systems, and Other Miscellaneous Mechanisms of Action. Mar. Drugs. 2017;15:273. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

