Chemically Tuning Resveratrol for the Effective Killing of Gram-Positive Pathogens
- PMID: 35621995
- PMCID: PMC9237828
- DOI: 10.1021/acs.jnatprod.1c01107
Chemically Tuning Resveratrol for the Effective Killing of Gram-Positive Pathogens
Abstract
In the era of antimicrobial resistance, the identification of new compounds with strong antimicrobial activity and the development of alternative therapies to fight drug-resistant bacteria are urgently needed. Here, we have used resveratrol, a safe and well-known plant-derived stilbene with poor antimicrobial properties, as a scaffold to design several new families of antimicrobials by adding different chemical entities at specific positions. We have characterized the mode of action of the most active compounds prepared and have examined their synergistic antibacterial activity in combination with traditional antibiotics. Some alkyl- and silyl-resveratrol derivatives show bactericidal activity against Gram-positive bacteria in the same low micromolar range of traditional antibiotics, with an original mechanism of action that combines membrane permeability activity with ionophore-related activities. No cross-resistance or antagonistic effect was observed with traditional antibiotics. Synergism was observed for some specific general-use antibiotics, such as aminoglycosides and cationic antimicrobial peptide antibiotics. No hemolytic activity was observed at the active concentrations or above, although some low toxicity against an MRC-5 cell line was noted.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
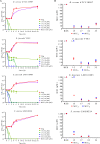
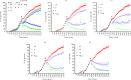
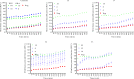
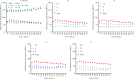

References
-
- Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics; WHO, 2017.
-
- WHO . Global Action Plan on Antimicrobial Resistance; World Health Organization, 2015. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

