Drug Interactions Affecting Oral Anticoagulant Use
- PMID: 35622425
- PMCID: PMC9308105
- DOI: 10.1161/CIRCEP.121.007956
Drug Interactions Affecting Oral Anticoagulant Use
Abstract
Oral anticoagulants (OACs) are medications commonly used in patients with atrial fibrillation and other cardiovascular conditions. Both warfarin and direct oral anticoagulants are susceptible to drug-drug interactions (DDIs). DDIs are an important cause of adverse drug reactions and exact a large toll on the health care system. DDI for warfarin mainly involve moderate to strong inhibitors/inducers of cytochrome P450 (CYP) 2C9, which is responsible for the elimination of the more potent S-isomer of warfarin. However, inhibitor/inducers of CYP3A4 and CYP1A2 may also cause DDI with warfarin. Recognition of these precipitating agents along with increased frequency of monitoring when these agents are initiated or discontinued will minimize the impact of warfarin DDI. Direct oral anticoagulants are mainly affected by medications strongly affecting the permeability glycoprotein (P-gp), and to a lesser extent, strong CYP3A4 inhibitors/inducers. Dabigatran and edoxaban are affected by P-gp modulation. Strong inducers of CYP3A4 or P-gp should be avoided in all patients taking direct oral anticoagulant unless previously proven to be otherwise safe. Simultaneous strong CYP3A4 and P-gp inhibitors should be avoided in patients taking apixaban and rivaroxaban. Concomitant antiplatelet/anticoagulant use confers additive risk for bleeding, but their combination is unavoidable in many cases. Minimizing duration of concomitant anticoagulant/antiplatelet therapy as indicated by evidence-based clinical guidelines is the best way to reduce the risk of bleeding.
Keywords: anticoagulants; apixaban; atrial fibrillation; glycoprotein; warfarin.
Figures

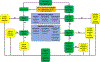
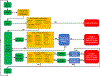
References
-
- Boehringer Ingelheim Pharmaceuticals. Pradaxa prescribing information. 2015;2015,
-
- Squibb Bristol-Myers. Eliquis prescribing information. 2014;2015,
-
- Sankyo Daiichi. Savaysa prescribing information. 2015;2015,
-
- Janssen Pharmaceuticals. Xarelto prescribing information. 2015;2015,
-
- Dai H, Zhang Q, Much AA, Maor E, Segev A, Beinart R, Adawi S, Lu Y, Bragazzi NL, Wu J. Global, regional, and national prevalence, incidence, mortality, and risk factors for atrial fibrillation, 1990–2017: Results from the global burden of disease study 2017. Eur Heart J Qual Care Clin Outcomes. 2020 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

