Clinical and Evolutionary Implications of Dynamic Coagulotoxicity Divergences in Bothrops (Lancehead Pit Viper) Venoms
- PMID: 35622544
- PMCID: PMC9148167
- DOI: 10.3390/toxins14050297
Clinical and Evolutionary Implications of Dynamic Coagulotoxicity Divergences in Bothrops (Lancehead Pit Viper) Venoms
Abstract
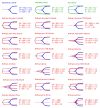
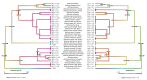
Despite coagulotoxicity being a primary weapon for prey capture by Bothrops species (lancehead pit vipers) and coagulopathy being a major lethal clinical effect, a genus-wide comparison has not been undertaken. To fill this knowledge gap, we used thromboelastography to compare 37 venoms, from across the full range of geography, taxonomy, and ecology, for their action upon whole plasma and isolated fibrinogen. Potent procoagulant toxicity was shown to be the main venom effect of most of the species tested. However, the most basal species (B. pictus) was strongly anticoagulant; this is consistent with procoagulant toxicity being a novel trait that evolved within Bothrops subsequent to their split from anticoagulant American pit vipers. Intriguingly, two of the arboreal species studied (B. bilineatus and B. taeniatus) lacked procoagulant venom, suggesting differential evolutionary selection pressures. Notably, some terrestrial species have secondarily lost the procoagulant venom trait: the Mogi Mirim, Brazil locality of B. alternatus; San Andres, Mexico locality of B. asper; B. diporus; and the São Roque of B. jararaca. Direct action on fibrinogen was extremely variable; this is consistent with previous hypotheses regarding it being evolutionary decoupled due to procoagulant toxicity being the primary prey-capture weapon. However, human patients live long enough for fibrinogen depletion to be clinically significant. The extreme variability may be reflective of antivenom variability, with these results thereby providing a foundation for such future work of clinical relevance. Similarly, the venom diversification trends relative to ecological niche will also be useful for integration with natural history data, to reconstruct the evolutionary pressures shaping the venoms of these fascinating snakes.
Keywords: Bothrops; anticoagulant; coagulopathy; fibrinogen; procoagulant; venom evolution.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

