Altered patterning of trisomy 21 interneuron progenitors
- PMID: 35623352
- PMCID: PMC9214050
- DOI: 10.1016/j.stemcr.2022.05.001
Altered patterning of trisomy 21 interneuron progenitors
Abstract
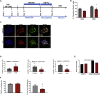
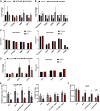
Individuals with Down syndrome (DS; Ts21), the most common genetic cause of intellectual disability, have smaller brains that reflect fewer neurons at pre- and post-natal stages, implicating impaired neurogenesis during development. Our stereological analysis of adult DS cortex indicates a reduction of calretinin-expressing interneurons. Using Ts21 human induced pluripotent stem cells (iPSCs) and isogenic controls, we find that Ts21 progenitors generate fewer COUP-TFII+ progenitors with reduced proliferation. Single-cell RNA sequencing of Ts21 progenitors confirms the altered specification of progenitor subpopulations and identifies reduced WNT signaling. Activation of WNT signaling partially restores the COUP-TFII+ progenitor population in Ts21, suggesting that altered WNT signaling contributes to the defective development of cortical interneurons in DS.
Keywords: Cortical development; Down syndrome; Neurogenesis; human; iPSCs; isogenic; neural differentiation; trisomy 21.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

