Molecular signatures written in bone proteins of 79 AD victims from Herculaneum and Pompeii
- PMID: 35624181
- PMCID: PMC9142588
- DOI: 10.1038/s41598-022-12042-6
Molecular signatures written in bone proteins of 79 AD victims from Herculaneum and Pompeii
Erratum in
-
Author Correction: Molecular signatures written in bone proteins of 79 AD victims from Herculaneum and Pompeii.Sci Rep. 2022 Jul 13;12(1):11888. doi: 10.1038/s41598-022-16362-5. Sci Rep. 2022. PMID: 35831554 Free PMC article. No abstract available.
Abstract
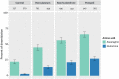
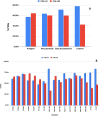
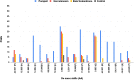
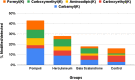
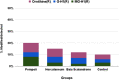
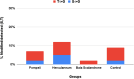
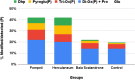
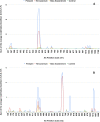
An extensive proteomic analysis was performed on a set of 12 bones of human victims of the eruption that in AD 79 rapidly buried Pompeii and Herculaneum, allowing the detection of molecular signatures imprinted in the surviving protein components. Bone collagen survived the heat of the eruption, bearing a piece of individual biological history encoded in chemical modifications. Here we show that the human bone proteomes from Pompeii are more degraded than those from the inhabitants of Herculaneum, despite the latter were exposed to temperatures much higher than those experienced in Pompeii. The analysis of the specimens from Pompeii shows lower content of non-collagenous proteins, higher deamidation level and higher extent of collagen modification. In Pompeii, the slow decomposition of victims' soft tissues in the natural dry-wet hydrogeological soil cycles damaged their bone proteome more than what was experienced at Herculaneum by the rapid vanishing of body tissues from intense heat, under the environmental condition of a permanent waterlogged burial context. Results herein presented are the first proteomic analyses of bones exposed to eruptive conditions, but also delivered encouraging results for potential biomarkers that might also impact future development of forensic bone proteomics.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Warren M. Ancient proteins tell their tales. Nature. 2019;570:433–436. - PubMed
-
- Kendall C, Eriksen AMH, Kontopoulos I, Collins MJ, Turner-Walker G. Diagenesis of archaeological bone and tooth. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2018;491:21–37.
-
- Mamede AP, Gonçalves D, Marques MPM, Batista de Carvalho LAE. Burned bones tell their own stories: a review of methodological approaches to assess heat-induced diagenesis. Appl. Spectrosc. Rev. 2018;53:603–635.
-
- Hedges REM. Bone diagenesis: an overview of processes. Archaeometry. 2002;44:319–328.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

