A truncating variant of RAD51B associated with primary ovarian insufficiency provides insights into its meiotic and somatic functions
- PMID: 35624308
- PMCID: PMC9751091
- DOI: 10.1038/s41418-022-01021-z
A truncating variant of RAD51B associated with primary ovarian insufficiency provides insights into its meiotic and somatic functions
Abstract
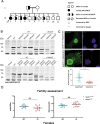
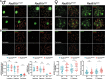
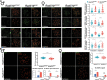
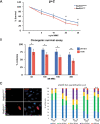
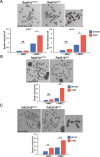
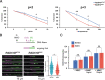
Primary ovarian insufficiency (POI) causes female infertility by abolishing normal ovarian function. Although its genetic etiology has been extensively investigated, most POI cases remain unexplained. Using whole-exome sequencing, we identified a homozygous variant in RAD51B -(c.92delT) in two sisters with POI. In vitro studies revealed that this variant leads to translation reinitiation at methionine 64. Here, we show that this is a pathogenic hypomorphic variant in a mouse model. Rad51bc.92delT/c.92delT mice exhibited meiotic DNA repair defects due to RAD51 and HSF2BP/BMRE1 accumulation in the chromosome axes leading to a reduction in the number of crossovers. Interestingly, the interaction of RAD51B-c.92delT with RAD51C and with its newly identified interactors RAD51 and HELQ was abrogated or diminished. Repair of mitomycin-C-induced chromosomal aberrations was impaired in RAD51B/Rad51b-c.92delT human and mouse somatic cells in vitro and in explanted mouse bone marrow cells. Accordingly, Rad51b-c.92delT variant reduced replication fork progression of patient-derived lymphoblastoid cell lines and pluripotent reprogramming efficiency of primary mouse embryonic fibroblasts. Finally, Rad51bc.92delT/c.92delT mice displayed increased incidence of pituitary gland hyperplasia. These results provide new mechanistic insights into the role of RAD51B not only in meiosis but in the maintenance of somatic genome stability.
© 2022. The Author(s), under exclusive licence to ADMC Associazione Differenziamento e Morte Cellulare.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

