Safety, Pharmacodynamics, and Pharmacokinetics of P2X3 Receptor Antagonist Eliapixant (BAY 1817080) in Healthy Subjects: Double-Blind Randomized Study
- PMID: 35624408
- PMCID: PMC9349145
- DOI: 10.1007/s40262-022-01126-1
Safety, Pharmacodynamics, and Pharmacokinetics of P2X3 Receptor Antagonist Eliapixant (BAY 1817080) in Healthy Subjects: Double-Blind Randomized Study
Abstract
Background and objective: There is no licensed treatment for refractory chronic cough; off-label therapies have limited efficacy and can produce adverse effects. Excessive adenosine triphosphate signaling via P2X3 receptors is implicated in refractory chronic cough, and selective P2X3 receptor antagonists such as eliapixant (BAY 1817080) are under investigation. The objective of the study was to investigate the safety and tolerability of ascending repeated oral doses of eliapixant in healthy volunteers.
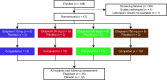
Methods: We conducted a repeated-dose, double-blind, randomized, placebo-controlled study in 47 healthy male individuals. Subjects received repeated twice-daily ascending oral doses of eliapixant (10, 50, 200, and 750 mg) or placebo for 2 weeks. The primary outcome was frequency and severity of adverse events. Other outcomes included pharmacokinetics and evaluation of taste disturbances, which have occurred with the less selective P2X3 receptor antagonist gefapixant.
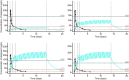
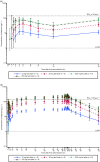
Results: Peak plasma concentrations of eliapixant were reached 3-4 h after administration of the first and subsequent doses. With multiple dosing, steady-state plasma concentrations were reached after ~ 6 days, and plasma concentrations predicted to achieve ≥ 80% P2X3 receptor occupancy (the level required for efficacy) were reached at 200 and 750 mg. Increases in plasma concentrations with increasing doses were less than dose proportional. After multiple dosing, mean plasma concentrations of eliapixant showed low peak-trough fluctuations and were similar for 200- and 750-mg doses. Eliapixant was well tolerated with a low incidence of taste-related adverse events.
Conclusions: Eliapixant (200 and 750 mg) produced plasma concentrations that cover the predicted therapeutic threshold over 24 h, with good safety and tolerability. These results enabled eliapixant to progress to clinical trials in patients with refractory chronic cough.
Clinical trial registration: Clinicaltrials.gov: NCT03310645 (initial registration: 16 October, 2017).
Plain language summary
There are few effective treatments for patients with a long-term (chronic) cough. It is thought that chronic cough is caused by nerves becoming oversensitive, wrongly causing a cough when there is no need. We tested a new drug called eliapixant in 47 healthy men. Eliapixant reduces the excessive nerve signaling responsible for chronic cough. We looked for side effects of eliapixant and measured how it behaves in the body. In particular we looked for side effects relating to the sense of taste because gefapixant, a similar drug to eliapixant, can affect taste. Participants took one of four eliapixant doses or a placebo twice daily for 2 weeks. The highest levels of eliapixant in the blood were seen 3–4 h after taking the drug, and stable concentrations were seen after about 6 days. At the two highest doses, eliapixant reached concentrations in the body that should be high enough to work in patients with chronic cough. Side effects were generally similar between eliapixant and placebo. Taste-related side effects were mild and went away without needing treatment. The positive results of this study meant that eliapixant could be tested in patients with chronic cough.
© 2022. The Author(s).
Conflict of interest statement
Christian Friedrich, Klaus Francke, Christian Scheerans, Stefan Klein, and Lueder Fels are employees of Bayer AG, Berlin, Germany. Isabella Gashaw was an employee of Bayer AG, Berlin, Germany, when the study was planned, conducted, and analyzed. Alyn Morice reports grants, personal fees, non-financial support, and other from Bayer AG, and grants, personal fees, non-financial support, and other from Bayer US, during the conduct of the study; personal fees and non-financial support from AstraZeneca, personal fees, non-financial support, and other from Bellus Health, personal fees and non-financial support from Boehringer Ingelheim, personal fees and non-financial support from Chiesi Ltd, grants, personal fees, and non-financial support from GlaxoSmithKline, grants, personal fees, non-financial support, and other from Merck Sharp & Dohme Corp., grants from Menlo Therapeutics, grants, personal fees, and other from NeRRe Therapeutics, grants, personal fees, and non-financial support from Phillips Respironics, grants, personal fees, and non-financial support from Respivant Sciences, Inc., and grants, personal fees, non-financial support, and other from Sanofi, outside the submitted work. Jaclyn A. Smith reports grants and personal fees from Bayer AG during the conduct of the study, personal fees from Algernon Pharmaceuticals, AstraZeneca, and Boehringer Ingelheim, grants and personal fees from Axalbion, Bellus Health, GlaxoSmithKline, Menlo Therapeutics, Merck Sharp & Dohme Corp., NeRRe Therapeutics, Nocion Therapeutics, and Shionogi Inc., outside the submitted work. The VitaloJAK algorithm has been licensed by Manchester University Foundation Trust and the University of Manchester to Vitalograph Ltd. and Vitalograph Ireland (Ltd.). Manchester University Foundation Trust receives royalties, which may be shared with the clinical division in which Jaclyn A. Smith works. Thomas Hummel reports grants from aspUraclip, Berlin, Germany, Smell and Taste Laboratory, Geneva, Switzerland, Sony, Stuttgart, Germany, and Takasago, Paris, France, and personal fees from Frequency Therapeutics, Farmington, CT, USA and Baiafoods, Madrid, Spain, outside the submitted work.
Figures

References
-
- Prado FC, Araldi D, Vieira AS, Oliveira-Fusaro MC, Tambeli CH, Parada CA. Neuronal P2X3 receptor activation is essential to the hyperalgesia induced by prostaglandins and sympathomimetic amines released during inflammation. Neuropharmacology. 2013;67:252–258. doi: 10.1016/j.neuropharm.2012.11.011. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

