An Antioxidant Supplement Function Exploration: Rescue of Intestinal Structure Injury by Mannan Oligosaccharides after Aeromonas hydrophila Infection in Grass Carp (Ctenopharyngodon idella)
- PMID: 35624670
- PMCID: PMC9137958
- DOI: 10.3390/antiox11050806
An Antioxidant Supplement Function Exploration: Rescue of Intestinal Structure Injury by Mannan Oligosaccharides after Aeromonas hydrophila Infection in Grass Carp (Ctenopharyngodon idella)
Abstract
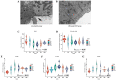
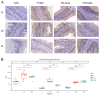
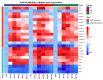
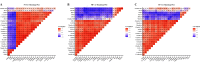
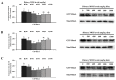
Mannan oligosaccharides (MOS) are a type of functional oligosaccharide which have received increased attention because of their beneficial effects on fish intestinal health. However, intestinal structural integrity is a necessary prerequisite for intestinal health. This study focused on exploring the protective effects of dietary MOS supplementation on the grass carp’s (Ctenopharyngodon idella) intestinal structural integrity (including tight junction (TJ) and adherent junction (AJ)) and its related signalling molecule mechanism. A total of 540 grass carp (215.85 ± 0.30 g) were fed six diets containing graded levels of dietary MOS supplementation (0, 200, 400, 600, 800 and 1000 mg/kg) for 60 days. Subsequently, a challenge test was conducted by injection of Aeromonas hydrophila for 14 days. We used ELISA, spectrophotometry, transmission electron microscope, immunohistochemistry, qRT-PCR and Western blotting to determine the effect of dietary MOS supplementation on intestinal structural integrity and antioxidant capacity. The results revealed that dietary MOS supplementation protected the microvillus of the intestine; reduced serum diamine oxidase and d-lactate levels (p < 0.05); enhanced intestinal total antioxidant capacity (p < 0.01); up-regulated most intestinal TJ and AJ mRNA levels; and decreased GTP-RhoA protein levels (p < 0.01). In addition, we also found several interesting results suggesting that MOS supplementation has no effects on ZO-2 and Claudin-15b. Overall, these findings suggested that dietary MOS supplementation could protect intestinal ultrastructure, reduce intestinal mucosal permeability and maintain intestinal structural integrity via inhibiting MLCK and RhoA/ROCK signalling pathways.
Keywords: adherent junction; antioxidant capacity; intestine; permeability; tight junction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- The Nutrition Division . Probiotics in Food: Health and Nutritional Properties and Guidelines for Evaluation. Food and Agriculture Organization of the United Nations; Rome, Italy: World Health Organization; Geneva, Switzerland: 2006. Report of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria, Cordoba, Argentina, 1–4 October 2001; Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food, London, Ontario, Canada, 30 April–1 May 2002.
-
- Islam S.M.M., Rohani M.F., Shahjahan M. Probiotic yeast enhances growth performance of Nile tilapia (Oreochromis niloticus) through morphological modifications of intestine. Aquac. Rep. 2021;21:100800. doi: 10.1016/j.aqrep.2021.100800. - DOI
-
- Nathanailides C., Kolygas M., Choremi K., Mavraganis T., Gouva E., Vidalis K., Athanassopoulou F. Probiotics Have the Potential to Significantly Mitigate the Environmental Impact of Freshwater Fish Farms. Fishes. 2021;6:76. doi: 10.3390/fishes6040076. - DOI
-
- Iorizzo M., Albanese G., Letizia F., Testa B., Tremonte P., Vergalito F., Lombardi S.J., Succi M., Coppola R., Sorrentino E. Probiotic Potentiality from Versatile Lactiplantibacillus plantarum Strains as Resource to Enhance Freshwater Fish Health. Microorganisms. 2022;10:463. doi: 10.3390/microorganisms10020463. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

