Metabolic Profiling, Chemical Composition, Antioxidant Capacity, and In Vivo Hepato- and Nephroprotective Effects of Sonchus cornutus in Mice Exposed to Cisplatin
- PMID: 35624682
- PMCID: PMC9137627
- DOI: 10.3390/antiox11050819
Metabolic Profiling, Chemical Composition, Antioxidant Capacity, and In Vivo Hepato- and Nephroprotective Effects of Sonchus cornutus in Mice Exposed to Cisplatin
Abstract
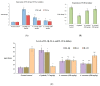
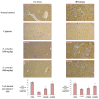
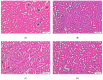
Sonchus cornutus (Asteraceae) is a wild. edible plant that represents a plentiful source of polyphenolic compounds. For the first time, the metabolic analysis profiling demonstrated the presence of anthocyanidin glycosides, coumarins, flavonoids and their corresponding glycosides, and phenolic acids. The total phenolic compounds were determined to be 206.28 ± 14.64 mg gallic acid equivalent/gm, while flavonoids were determined to be 45.56 ± 1.78 mg quercetin equivalent/gm. The crude extract of S. cornutus exhibited a significant 1,1-diphenyl-2-picrylhydrazyl free radical scavenging effect with half-maximal inhibitory concentration (IC50) of 16.10 ± 2.14 µg/mL compared to ascorbic acid as a standard (10.64 ± 0.82 µg/mL). In vitro total antioxidant capacity and ferric reducing power capacity assays revealed a promising reducing potential of S. cornutus extract. Therefore, the possible protective effects of S. cornutus against hepatic and renal toxicity induced by cisplatin in experimental mice were investigated. S. cornutus significantly ameliorated the cisplatin-induced disturbances in liver and kidney functions and oxidative stress, decreased MDA, ROS, and NO levels, and restored CAT and SOD activities. Besides, it reversed cisplatin-driven upregulation in inflammatory markers, including iNOS, IL-6, and IL-1β levels and NF-κB and TNF-α expression, and elevated anti-inflammatory IL-10 levels and Nrf2 expression. Additionally, the extract mitigated cisplatin alteration in apoptotic (Bax and caspase-3) and anti-apoptotic (Bcl-2) proteins. Interestingly, hepatic, and renal histopathology revealed the protective impacts of S. cornutus against cisplatin-induced pathological changes. Our findings guarantee a protective effect of S. cornutus against cisplatin-induced hepatic and renal damage via modulating oxidative stress, inflammation, and apoptotic pathways.
Keywords: Sonchus cornutus; apoptosis; drug discovery; hepatotoxicity; industries development; inflammation; nephrotoxicity; oxidative stress; polyphenolic compounds.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Singh N., Magotra R., Sharma A.K., Ahmed M., Khajuria V. Effect of cisplatin on liver of male albino rats. J. Evol. Med. Dent. Sci. 2015;4:8993–8998. doi: 10.14260/jemds/2015/1305. - DOI
-
- Abouzed T., Soliman M., Khatab S., Elgazzar A., Gouda W., Dorghamm D. The protective impacts of Spriulina platensis against cisplatin-induced renal injury through the regulation of oxidative stress, pro-inflammatory cytokines and Bax/Bcl2 expression cascade. Toxicol. Res. 2022;11:169–178. doi: 10.1093/toxres/tfab128. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

