Anti-Inflammatory Effect of Resveratrol Derivatives via the Downregulation of Oxidative-Stress-Dependent and c-Src Transactivation EGFR Pathways on Rat Mesangial Cells
- PMID: 35624699
- PMCID: PMC9138040
- DOI: 10.3390/antiox11050835
Anti-Inflammatory Effect of Resveratrol Derivatives via the Downregulation of Oxidative-Stress-Dependent and c-Src Transactivation EGFR Pathways on Rat Mesangial Cells
Abstract
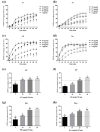
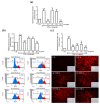
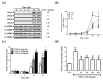
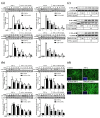
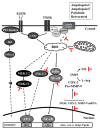
In Taiwan, the root extract of Vitis thunbergii Sieb. et Zucc. (Vitaceae, VT) is rich in stilbenes, with resveratrol (Res) and its derivatives being the most abundant. Previously, we showed that the effect of Res derivatives against tumor necrosis factor-α (TNF-α)-stimulated inflammatory responses occurs via cPLA2/COX-2/PGE2 inhibition. This study compared and explored the underlying anti-inflammatory pharmacological mechanisms. Before stimulation with TNF-α, RMCs were treated with/without pharmacological inhibitors of specific protein kinases. The expression of inflammatory mediators was determined by Western blotting, gelatin zymography, real-time PCR, and luciferase assay. Cellular and mitochondrial ROS were measured by H2DHFDA or DHE and MitoSOX™ Red staining, respectively. The RNS level was indirectly measured by Griess reagent assay. Kinase activation and association were assayed by immunoprecipitation followed by Western blotting. TNF-α binding to TNFR recruited Rac1 and p47phox, thus activating the NAPDH oxidase-dependent MAPK and NF-κB pathways. The TNF-α-induced NF-κB activation via c-Src-driven ROS was independent from the EGFR signaling pathway. The anti-inflammatory effects of Res derivatives occurred via the inhibition of ROS derived from mitochondria and NADPH oxidase; RNS derived from iNOS; and the activation of the ERK1/2, JNK1/2, and NF-κB pathways. Overall, this study provides an understanding of the various activities of Res derivatives and their pharmacological mechanisms. In the future, the application of the active molecules of VT to health foods and medicine in Taiwan may increase.
Keywords: Res derivatives; anti-inflammation; anti-oxidation; transactivation EGFR.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

