NiONP-Induced Oxidative Stress and Mitochondrial Impairment in an In Vitro Pulmonary Vascular Cell Model Mimicking Endothelial Dysfunction
- PMID: 35624710
- PMCID: PMC9137840
- DOI: 10.3390/antiox11050847
NiONP-Induced Oxidative Stress and Mitochondrial Impairment in an In Vitro Pulmonary Vascular Cell Model Mimicking Endothelial Dysfunction
Abstract
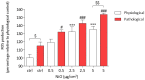
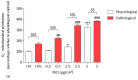
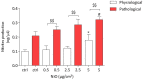
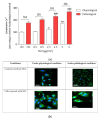
The development and use of nanomaterials, especially of nickel oxide nanoparticles (NiONPs), is expected to provide many benefits but also has raised concerns about the potential human health risks. Inhaled NPs are known to exert deleterious cardiovascular side effects, including pulmonary hypertension. Consequently, patients with pulmonary hypertension (PH) could be at increased risk for morbidity. The objective of this study was to compare the toxic effects of NiONPs on human pulmonary artery endothelial cells (HPAEC) under physiological and pathological conditions. The study was conducted with an in vitro model mimicking the endothelial dysfunction observed in PH. HPAEC were cultured under physiological (static and normoxic) or pathological (20% cycle stretch and hypoxia) conditions and exposed to NiONPs (0.5-5 μg/cm2) for 4 or 24 h. The following endpoints were studied: (i) ROS production using CM-H2DCF-DA and MitoSOX probes, (ii) nitrite production by the Griess reaction, (iii) IL-6 secretion by ELISA, (iv) calcium signaling with a Fluo-4 AM probe, and (v) mitochondrial dysfunction with TMRM and MitoTracker probes. Our results evidenced that under pathological conditions, ROS and nitrite production, IL-6 secretions, calcium signaling, and mitochondria alterations increased compared to physiological conditions. Human exposure to NiONPs may be associated with adverse effects in vulnerable populations with cardiovascular risks.
Keywords: calcium; cyclic stretch; endothelial dysfunction; human pulmonary artery endothelial cells; mitochondria alteration; nickel oxide nanoparticles; reactive oxygen species.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
NiONPs-induced alteration in calcium signaling and mitochondrial function in pulmonary artery endothelial cells involves oxidative stress and TRPV4 channels disruption.Nanotoxicology. 2022 Feb;16(1):29-51. doi: 10.1080/17435390.2022.2030821. Epub 2022 Jan 28. Nanotoxicology. 2022. PMID: 35090355
-
Effects of FW2 Nanoparticles Toxicity in a New In Vitro Pulmonary Vascular Cells Model Mimicking Endothelial Dysfunction.Cardiovasc Toxicol. 2022 Jan;22(1):14-28. doi: 10.1007/s12012-021-09679-6. Epub 2021 Sep 15. Cardiovasc Toxicol. 2022. PMID: 34524626
-
Involvement of oxidative stress and calcium signaling in airborne particulate matter - induced damages in human pulmonary artery endothelial cells.Toxicol In Vitro. 2017 Dec;45(Pt 3):340-350. doi: 10.1016/j.tiv.2017.07.001. Epub 2017 Jul 5. Toxicol In Vitro. 2017. PMID: 28688989
-
Fetal programming of atherosclerosis: possible role of the mitochondria.Eur J Obstet Gynecol Reprod Biol. 2010 Apr;149(2):127-30. doi: 10.1016/j.ejogrb.2009.12.005. Epub 2010 Jan 6. Eur J Obstet Gynecol Reprod Biol. 2010. PMID: 20053495 Review.
-
A Brief Overview of Nitric Oxide and Reactive Oxygen Species Signaling in Hypoxia-Induced Pulmonary Hypertension.Adv Exp Med Biol. 2017;967:71-81. doi: 10.1007/978-3-319-63245-2_6. Adv Exp Med Biol. 2017. PMID: 29047082 Free PMC article. Review.
Cited by
-
The pulmonary effects of nickel-containing nanoparticles: Cytotoxicity, genotoxicity, carcinogenicity, and their underlying mechanisms.Environ Sci Nano. 2024 May 1;11(5):1817-1846. doi: 10.1039/d3en00929g. Epub 2024 Mar 21. Environ Sci Nano. 2024. PMID: 38984270 Free PMC article.
-
Heavy Metal Exposure-induced Cardiovascular Diseases: Molecular Mechanisms and Therapeutic Role of Antioxidants.Curr Med Chem. 2025;32(17):3438-3465. doi: 10.2174/0109298673307446240514064253. Curr Med Chem. 2025. PMID: 38757322 Review.
-
Endothelial dysfunction in cardiovascular diseases: mechanisms and in vitro models.Mol Cell Biochem. 2025 Aug;480(8):4671-4695. doi: 10.1007/s11010-025-05289-w. Epub 2025 Apr 21. Mol Cell Biochem. 2025. PMID: 40259179 Free PMC article. Review.
-
Understanding the Mechanism of Cardiotoxicity Induced by Nanomaterials: A Comprehensive Review.Small Sci. 2025 Feb 20;5(5):2400498. doi: 10.1002/smsc.202400498. eCollection 2025 May. Small Sci. 2025. PMID: 40395348 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

