OPDAylation of Thiols of the Redox Regulatory Network In Vitro
- PMID: 35624719
- PMCID: PMC9137622
- DOI: 10.3390/antiox11050855
OPDAylation of Thiols of the Redox Regulatory Network In Vitro
Abstract
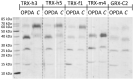
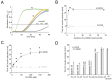
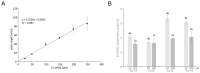
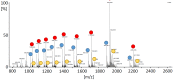
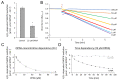
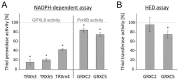
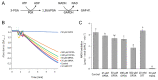
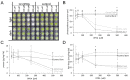
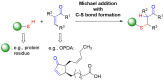
cis-(+)-12-Oxophytodienoic acid (OPDA) is a reactive oxylipin produced by catalytic oxygenation of polyunsaturated α-linolenic acid (18:3 (ω - 3)) in the chloroplast. Apart from its function as precursor for jasmonic acid synthesis, OPDA serves as a signaling molecule and regulator on its own, namely by tuning enzyme activities and altering expression of OPDA-responsive genes. A possible reaction mechanism is the covalent binding of OPDA to thiols via the addition to the C=C double bond of its α,β-unsaturated carbonyl group in the cyclopentenone ring. The reactivity allows for covalent modification of accessible cysteinyl thiols in proteins. This work investigated the reaction of OPDA with selected chloroplast and cytosolic thioredoxins (TRX) and glutaredoxins (GRX) of Arabidopsis thaliana. OPDA reacted with TRX and GRX as detected by decreased m-PEG maleimide binding, consumption of OPDA, reduced ability for insulin reduction and inability to activate glyceraldehyde-3-phosphate dehydrogenase and regenerate glutathione peroxidase (GPXL8), and with lower efficiency, peroxiredoxin IIB (PRXIIB). OPDAylation of certain protein thiols occurs quickly and efficiently in vitro and is a potent post-translational modification in a stressful environment.
Keywords: Arabidopsis thaliana; oxylipin; posttranslational modification; thiol; thioredoxin.
Conflict of interest statement
The authors declare that they encounter no conflict of interest.
Figures




Similar articles
-
The In Vitro Interaction of 12-Oxophytodienoic Acid and Related Conjugated Carbonyl Compounds with Thiol Antioxidants.Biomolecules. 2021 Mar 18;11(3):457. doi: 10.3390/biom11030457. Biomolecules. 2021. PMID: 33803875 Free PMC article.
-
12-Oxophytodienoic Acid Reductase 3 (OPR3) Functions as NADPH-Dependent α,β-Ketoalkene Reductase in Detoxification and Monodehydroascorbate Reductase in Redox Homeostasis.Plant Cell Physiol. 2020 Mar 1;61(3):584-595. doi: 10.1093/pcp/pcz226. Plant Cell Physiol. 2020. PMID: 31834385
-
Biochemical Characterization of 13-Lipoxygenases of Arabidopsis thaliana.Int J Mol Sci. 2021 Sep 23;22(19):10237. doi: 10.3390/ijms221910237. Int J Mol Sci. 2021. PMID: 34638573 Free PMC article.
-
The function of the oxylipin 12-oxophytodienoic acid in cell signaling, stress acclimation, and development.J Exp Bot. 2018 Nov 26;69(22):5341-5354. doi: 10.1093/jxb/ery316. J Exp Bot. 2018. PMID: 30169821 Review.
-
The thioredoxin antioxidant system.Free Radic Biol Med. 2014 Jan;66:75-87. doi: 10.1016/j.freeradbiomed.2013.07.036. Epub 2013 Jul 27. Free Radic Biol Med. 2014. PMID: 23899494 Review.
Cited by
-
Oxylipins and Reactive Carbonyls as Regulators of the Plant Redox and Reactive Oxygen Species Network under Stress.Antioxidants (Basel). 2023 Mar 27;12(4):814. doi: 10.3390/antiox12040814. Antioxidants (Basel). 2023. PMID: 37107189 Free PMC article. Review.
-
Organelles and phytohormones: a network of interactions in plant stress responses.J Exp Bot. 2022 Nov 19;73(21):7165-7181. doi: 10.1093/jxb/erac384. J Exp Bot. 2022. PMID: 36169618 Free PMC article. Review.
-
Post-Translational Modifications to Cysteine Residues in Plant Proteins and Their Impact on the Regulation of Metabolism and Signal Transduction.Int J Mol Sci. 2024 Sep 12;25(18):9845. doi: 10.3390/ijms25189845. Int J Mol Sci. 2024. PMID: 39337338 Free PMC article. Review.
-
OPDA/dn-OPDA actions: biosynthesis, metabolism, and signaling.Plant Cell Rep. 2024 Aug 2;43(8):206. doi: 10.1007/s00299-024-03286-9. Plant Cell Rep. 2024. PMID: 39093416 Review.
References
-
- Monte I., Zamarreno A.M., Hamberg M., Lassueur S., Reymond P., Weiss S., Stintzi A., Schaller A., Porzel A., Garcia-Mina J.M., et al. An OPR3-independent pathway uses 4,5-didehydrojasmonate for jasmonate synthesis. Nat. Chem. Biol. 2018;14:171–178. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

