The Therapeutic and Prophylactic Potential of Quercetin against COVID-19: An Outlook on the Clinical Studies, Inventive Compositions, and Patent Literature
- PMID: 35624740
- PMCID: PMC9137692
- DOI: 10.3390/antiox11050876
The Therapeutic and Prophylactic Potential of Quercetin against COVID-19: An Outlook on the Clinical Studies, Inventive Compositions, and Patent Literature
Abstract
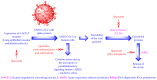
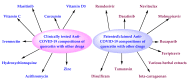
Quercetin is a phenolic flavonol compound with established antioxidant, anti-inflammatory, and immuno-stimulant properties. Recent studies demonstrate the potential of quercetin against COVID-19. This article highlighted the prophylactic/therapeutic potential of quercetin against COVID-19 in view of its clinical studies, inventions, and patents. The literature for the subject matter was collected utilizing different databases, including PubMed, Sci-Finder, Espacenet, Patentscope, and USPTO. Clinical studies expose the potential of quercetin monotherapy, and also its combination therapy with other compounds, including zinc, vitamin C, curcumin, vitamin D3, masitinib, hydroxychloroquine, azithromycin, and ivermectin. The patent literature also examines claims that quercetin containing nutraceuticals, pharmaceuticals, and dietary supplements, alone or in combination with other drugs/compounds, including favipiravir, remdesivir, molnupiravir, navitoclax, dasatinib, disulfiram, rucaparib, tamarixin, iota-carrageenan, and various herbal extracts (aloe, poria, rosemary, and sphagnum) has potential for use against COVID-19. The literature reveals that quercetin exhibits anti-COVID-19 activity because of its inhibitory effect on the expression of the human ACE2 receptors and the enzymes of SARS-CoV-2 (MPro, PLPro, and RdRp). The USFDA designated quercetin as a "Generally Recognized as Safe" substance for use in the food and beverage industries. It is also an inexpensive and readily available compound. These facts increase the possibility and foreseeability of making novel and economical drug combinations containing quercetin to prevent/treat COVID-19. Quercetin is an acidic compound and shows metabolic interaction with some antivirals, antibiotics, and anti-inflammatory agents. Therefore, the physicochemical and metabolic drug interactions between quercetin and the combined drugs/compounds must be better understood before developing new compositions.
Keywords: COVID-19; antioxidant; clinical trial; invention; patent; quercetin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Coronavirus Disease (COVID-19) Pandemic. [(accessed on 4 March 2022)]. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

