Thioredoxin-1 Ameliorates Oxygen-Induced Retinopathy in Newborn Mice through Modulation of Proinflammatory and Angiogenic Factors
- PMID: 35624763
- PMCID: PMC9137876
- DOI: 10.3390/antiox11050899
Thioredoxin-1 Ameliorates Oxygen-Induced Retinopathy in Newborn Mice through Modulation of Proinflammatory and Angiogenic Factors
Abstract
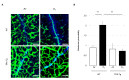
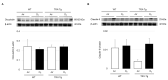
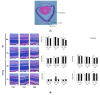
Oxygen-induced retinopathy (OIR) is an animal model for retinopathy of prematurity, which is a leading cause of blindness in children. Thioredoxin-1 (TRX) is a small redox protein that has cytoprotective and anti-inflammatory properties in response to oxidative stress. The purpose of this study was to determine the effect of TRX on OIR in newborn mice. From postnatal day 7, C57BL/6 wild type (WT) and TRX transgenic (TRX-Tg) mice were exposed to either 21% or 75% oxygen for 5 days. Avascular and neovascular regions of the retinas were investigated using fluorescence immunostaining. Fluorescein isothiocyanate-dextran and Hoechst staining were used to measure retinal vascular leakage. mRNA expression levels of proinflammatory and angiogenic factors were analyzed using quantitative polymerase chain reaction. Retinal histological changes were detected using immunohistochemistry. In room air, the WT mice developed well-organized retinas. In contrast, exposing WT newborn mice to hyperoxia hampered retinal development, increasing the retinal avascular and neovascular areas. After hyperoxia exposure, TRX-Tg mice had enhanced retinal avascularization compared with WT mice. TRX-Tg mice had lower retinal neovascularization and retinal permeability during recovery from hyperoxia compared with WT mice. In the early stages after hyperoxia exposure, VEGF-A and CXCL-2 expression levels decreased, while IL-6 expression levels increased in WT newborn mice. Conversely, no differences in gene expressions were observed in the TRX-Tg mouse retina. IGF-1 and Angpt1 levels did not decrease during recovery from hyperoxia in TRX-Tg newborn mice. As a result, overexpression of TRX improves OIR in newborn mice by modulating proinflammatory and angiogenic factors.
Keywords: angiogenic factor; hyperoxia; newborn mouse; oxygen-induced retinopathy; retinal blood vessel; retinopathy of prematurity; thioredoxin-1.
Conflict of interest statement
The authors have no affiliations with or involvement in any organization or entity with any financial or nonfinancial interest in the subject matter or materials discussed in this manuscript.
Figures





References
Grants and funding
- 29-F-1-06/Saitama Medical University Young Doctors Educational Grant
- 01-F-1-03/Saitama Medical University Young Doctors Educational Grant
- 18-B-1-11/Saitama Medical University Internal Research Grant
- 19K08259/Grants-in-Aid for Scientific Research (KAKENHI) from the Japan Society for the Promotion of Science
- 2019/Ochiai Memorial Award Research Grant
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

