Occurrence of Fibropapillomatosis in Green Turtles (Chelonia mydas) in Relation to Environmental Changes in Coastal Ecosystems in Texas and Florida: A Retrospective Study
- PMID: 35625082
- PMCID: PMC9137486
- DOI: 10.3390/ani12101236
Occurrence of Fibropapillomatosis in Green Turtles (Chelonia mydas) in Relation to Environmental Changes in Coastal Ecosystems in Texas and Florida: A Retrospective Study
Abstract
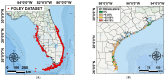
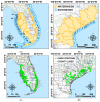
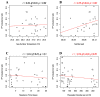
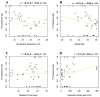
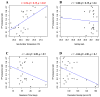
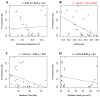
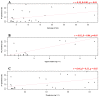
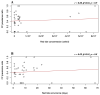
Fibropapillomatosis is a neoplastic disease of marine turtles, with green turtles (Chelonia mydas) being the most affected species. Fibropapillomatosis causes debilitating tumor growths on soft tissues and internal organs, often with lethal consequences. Disease incidence has been increasing in the last few decades and the reason is still uncertain. The potential viral infectious agent of Fibropapillomatosis, chelonid herpesvirus 5, has been co-evolving with its sea turtle host for millions of years and no major mutation linked with increased disease occurrence has been detected. Hence, frequent outbreaks in recent decades are likely attributable to external drivers such as large-scale anthropogenic changes in the green turtle coastal marine ecosystem. This study found that variations in sea surface temperature, salinity, and nutrient effluent discharge from nearby rivers were correlated with an increased incidence of the disease, substantiating that these may be among the significant environmental drivers impacting Fibropapillomatosis prevalence. This study offers data and insight on the need to establish a baseline of environmental factors which may drive Fibropapillomatosis and its clinical exacerbation. We highlight the multifactorial nature of this disease and support the inclusion of interdisciplinary work in future Fibropapillomatosis research efforts.
Keywords: coastal waters; ecosystem change; environmental impact; marine ecology; turtles; viruses; wildlife diseases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Duffy D.J., Schnitzler C., Karpinski L., Thomas R., Whilde J., Eastman C., Yang C., Krstic A., Rollinson D., Zirkelbach B., et al. Sea turtle fibropapilloma tumors share genomic drivers and therapeutic vulnerabilities with human cancers. Commun. Biol. 2018;1:1–13. doi: 10.1038/s42003-018-0059-x. - DOI - PMC - PubMed
-
- Smith G.M., Coates C.W. Fibro-epithelial growths of the skin in large marine turtles, Chelonia mydas (Linnaeus) Zoologica. 1938;23:93–96. doi: 10.5962/p.203654. - DOI
-
- Hirama S., Ehrhart L.M. Description, prevalence and severity of green turtle fibropapillomatosis in three developmental habitats on the east coast of Florida. Florida Sci. 2007;70:435–448.
LinkOut - more resources
Full Text Sources

