The Influence of Antibiotic Resistance on Innate Immune Responses to Staphylococcus aureus Infection
- PMID: 35625186
- PMCID: PMC9138074
- DOI: 10.3390/antibiotics11050542
The Influence of Antibiotic Resistance on Innate Immune Responses to Staphylococcus aureus Infection
Abstract
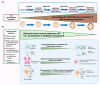
Staphylococcus aureus (S. aureus) causes a broad range of infections and is associated with significant morbidity and mortality. S. aureus produces a diverse range of cellular and extracellular factors responsible for its invasiveness and ability to resist immune attack. In recent years, increasing resistance to last-line anti-staphylococcal antibiotics daptomycin and vancomycin has been observed. Resistant strains of S. aureus are highly efficient in invading a variety of professional and nonprofessional phagocytes and are able to survive inside host cells. Eliciting immune protection against antibiotic-resistant S. aureus infection is a global challenge, requiring both innate and adaptive immune effector mechanisms. Dendritic cells (DC), which sit at the interface between innate and adaptive immune responses, are central to the induction of immune protection against S. aureus. However, it has been observed that S. aureus has the capacity to develop further antibiotic resistance and acquire increased resistance to immunological recognition by the innate immune system. In this article, we review the strategies utilised by S. aureus to circumvent antibiotic and innate immune responses, especially the interaction between S. aureus and DC, focusing on how this relationship is perturbed with the development of antibiotic resistance.
Keywords: Staphylococcus aureus; antibiotic resistance; innate immunity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Fowler V.G., Jr., Boucher H.W., Corey G.R., Abrutyn E., Karchmer A.W., Rupp M.E., Levine D.P., Chambers H.F., Tally F.P., Vigliani G.A., et al. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N. Engl. J. Med. 2006;355:653–665. doi: 10.1056/NEJMoa053783. - DOI - PubMed
-
- Howden B.P., Davies J.K., Johnson P.D., Stinear T.P., Grayson M.L. Reduced vancomycin susceptibility in Staphylococcus aureus, including vancomycin-intermediate and heterogeneous vancomycin-intermediate strains: Resistance mechanisms, laboratory detection, and clinical implications. Clin. Microbiol. Rev. 2010;23:99–139. doi: 10.1128/CMR.00042-09. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

