Niclosamide as a Repurposing Drug against Corynebacterium striatum Multidrug-Resistant Infections
- PMID: 35625295
- PMCID: PMC9137567
- DOI: 10.3390/antibiotics11050651
Niclosamide as a Repurposing Drug against Corynebacterium striatum Multidrug-Resistant Infections
Abstract
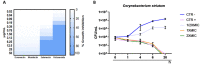
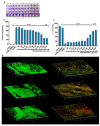
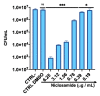
Corynebacterium striatum (C. striatum) is an emerging multidrug-resistant (MDR) pathogen associated with nosocomial infections. In this scenario, we screened the antimicrobial activity of the anthelmintic drugs doramectin, moxidectin, selamectin and niclosamide against 20 C. striatum MDR clinical isolates. Among these, niclosamide was the best performing drug against C. striatum. Niclosamide cytotoxicity was evaluated by a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay on immortalized human keratinocyte cells (HaCaT). After 20 h of treatment, the recorded 50% cytotoxic concentration (CC50) was 2.56 μg/mL. The antibacterial efficacy was determined via disc diffusion, broth microdilution method and time-killing. Against C. striatum, niclosamide induced a growth inhibitory area of 22 mm and the minimum inhibitory concentration that inhibits 90% of bacteria (MIC90) was 0.39 μg/mL, exhibiting bactericidal action. The biofilm biomass eradicating action was investigated through crystal violet (CV), MTT and confocal laser scanning microscopy (CLSM). Niclosamide affected the biofilm viability in a dose-dependent manner and degraded biomass by 55 and 49% at 0.39 μg/mL and 0.19 μg/mL. CLSM images confirmed the biofilm biomass degradation, showing a drastic reduction in cell viability. This study could promote the drug-repurposing of the anthelmintic FDA-approved niclosamide as a therapeutic agent to counteract the C. striatum MDR infections.
Keywords: Corynebacterium striatum; drug-repurposing; multidrug-resistant pathogen.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
-
- Folliero V., Franci G., Dell’Annunziata F., Giugliano R., Foglia F., Sperlongano R., De Filippis A., Finamore E., Galdiero M. Evaluation of Antibiotic Resistance and Biofilm Production among Clinical Strain Isolated from Medical Devices. Int. J. Microbiol. 2021;2021:9033278. doi: 10.1155/2021/9033278. - DOI - PMC - PubMed
-
- Verroken A., Bauraing C., Deplano A., Bogaerts P., Huang D., Wauters G., Glupczynski Y. Epidemiological Investigation of a Nosocomial Outbreak of Multidrug-Resistant Corynebacterium striatum at One Belgian University Hospital. Clin. Microbiol. Infect. 2014;20:44–50. doi: 10.1111/1469-0691.12197. - DOI - PubMed
-
- Oliveira A., Oliveira L.C., Aburjaile F., Benevides L., Tiwari S., Jamal S.B., Silva A., Figueiredo H.C.P., Ghosh P., Portela R.W., et al. Insight of Genus Corynebacterium: Ascertaining the Role of Pathogenic and Non-Pathogenic Species. Front. Microbiol. 2017;8:1937. doi: 10.3389/fmicb.2017.01937. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

