Advances towards Understanding the Mechanism of Action of the Hsp90 Complex
- PMID: 35625528
- PMCID: PMC9138868
- DOI: 10.3390/biom12050600
Advances towards Understanding the Mechanism of Action of the Hsp90 Complex
Abstract
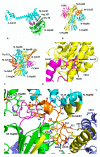
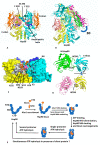
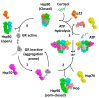
Hsp90 (Heat Shock Protein 90) is an ATP (Adenosine triphosphate) molecular chaperone responsible for the activation and maturation of client proteins. The mechanism by which Hsp90 achieves such activation, involving structurally diverse client proteins, has remained enigmatic. However, recent advances using structural techniques, together with advances in biochemical studies, have not only defined the chaperone cycle but have shed light on its mechanism of action. Hsp90 hydrolysis of ATP by each protomer may not be simultaneous and may be dependent on the specific client protein and co-chaperone complex involved. Surprisingly, Hsp90 appears to remodel client proteins, acting as a means by which the structure of the client protein is modified to allow its subsequent refolding to an active state, in the case of kinases, or by making the client protein competent for hormone binding, as in the case of the GR (glucocorticoid receptor). This review looks at selected examples of client proteins, such as CDK4 (cyclin-dependent kinase 4) and GR, which are activated according to the so-called 'remodelling hypothesis' for their activation. A detailed description of these activation mechanisms is paramount to understanding how Hsp90-associated diseases develop.
Keywords: ATPase; Aha1; Cdc37; Hsp90; chaperone; co-chaperone; heat shock proteins; immunophilins; kinase; mechanism; p23; steroid hormone receptor; structure.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Prodromou C., Morgan R.M.L. Tuning the ATPase Activity of Hsp90. In: Chakraborti S., Dhalla N.S., editors. Regulation of Ca2+-ATPases, V-ATPases and F-ATPases. Advances in Biochemistry in Health and Disease. Volume 14. Springer; Cham, Switzerland: 2016. pp. 469–490.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

