Advances in Visualizing Microglial Cells in Human Central Nervous System Tissue
- PMID: 35625531
- PMCID: PMC9138569
- DOI: 10.3390/biom12050603
Advances in Visualizing Microglial Cells in Human Central Nervous System Tissue
Abstract
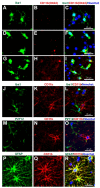
Neuroinflammation has recently been identified as a fundamentally important pathological process in most, if not all, CNS diseases. The main contributor to neuroinflammation is the microglia, which constitute the innate immune response system. Accurate identification of microglia and their reactivity state is therefore essential to further our understanding of CNS pathophysiology. Many staining techniques have been used to visualise microglia in rodent and human tissue, and immunostaining is currently the most frequently used. Historically, identification of microglia was predominantly based on morphological structure, however, recently there has been a reliance on selective antigen expression, and microglia-specific markers have been identified providing increased certainty that the cells observed are in fact microglia, rather than the similar yet distinct macrophages. To date, the most microglia-specific markers are P2Y12 and TMEM119. However, other microglia-related markers can also be useful for demonstrating activation state, phagocytic state, and for neuroimaging purposes in longitudinal studies. Overall, it is important to be aware of the microglia-selectivity issues of the various stains and immunomarkers used by researchers to distinguish microglia in CNS tissue to avoid misinterpretation.
Keywords: immunohistochemistry; immunostaining; microglia; neuroinflammation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Mammana S., Fagone P., Cavalli E., Basile M.S., Petralia M.C., Nicoletti F., Bramanti P., Mazzon E. The Role of Macrophages in Neuroinflammatory and Neurodegenerative Pathways of Alzheimer’s Disease, Amyotrophic Lateral Sclerosis, and Multiple Sclerosis: Pathogenetic Cellular Effectors and Potential Therapeutic Targets. Int. J. Mol. Sci. 2018;19:831. doi: 10.3390/ijms19030831. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

