Hyperthermia Enhances Efficacy of Chemotherapeutic Agents in Pancreatic Cancer Cell Lines
- PMID: 35625581
- PMCID: PMC9138677
- DOI: 10.3390/biom12050651
Hyperthermia Enhances Efficacy of Chemotherapeutic Agents in Pancreatic Cancer Cell Lines
Abstract
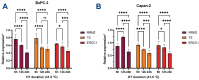
Chemotherapy (CT) is the standard care for advanced pancreatic ductal adenocarcinoma (PDAC); however, with limited efficacy. Hyperthermia (HT) treatment has been suggested as a sensitizer to improve outcomes. However, the direct effect of the HT and CT combination is not fully understood. Therefore, we aim to assess the direct cytotoxic effect of HT in PDAC cells as monotherapy or in combination with chemotherapeutics. Different temperatures (37-, 40.5-, 41-, and 41.5 °C) and durations (6-, 12-, and 24 h) were tested in PDAC cell lines (BxPC-3, Capan-1, Capan-2, PANC-1, and MIA-PaCa-2). Different concentrations of gemcitabine, 5-fluorouracil, and cisplatin were also tested in these conditions. The impact on cell metabolic activity was determined by an MTS assay. Enhancement of chemosensitivity was assessed by a reduction in half-maximal inhibitory concentration (IC50). HT and chemotherapeutics interactions were classified as antagonistic, additive, or synergistic using the combination index. HT inhibited cell proliferation in a cell type, temperature, and duration-dependent manner. The induction of apoptosis was seen after 6 h of HT treatment, eventually followed by secondary necrosis. The HT and CT combination led to an IC50 reduction of the tested CT. At 12 h of HT, this effect was between 25 to 90% and reached a 95% reduction at 24 h. The additive or synergistic effect was demonstrated in all cell lines and chemotherapeutics, although, again, this depended on cell type, duration, and temperature. HT is cytotoxic and enhances the therapeutic effectiveness of gemcitabine, 5-fluorouracil, and cisplatin on PDAC cells. This result was further confirmed by the decrease in the expression of RRM2, TS, and ERCC1 in BxPC-3 and Capan-2 cells. These observations warrant further study in specific subsets of PDAC patients to improve their clinical outcomes.
Keywords: 5-fluorouracil; anticancer therapy; cell proliferation; cisplatin; gemcitabine; thermal therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

