Exosomes Derived from BM-MSCs Mitigate the Development of Chronic Kidney Damage Post-Menopause via Interfering with Fibrosis and Apoptosis
- PMID: 35625591
- PMCID: PMC9138582
- DOI: 10.3390/biom12050663
Exosomes Derived from BM-MSCs Mitigate the Development of Chronic Kidney Damage Post-Menopause via Interfering with Fibrosis and Apoptosis
Abstract
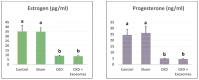
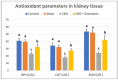
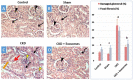
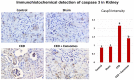
The rate of chronic kidney disease (CKD) is increasing globally, and it is caused by continuous damage to kidney tissue. With time the renal damage becomes irreversible, leading to CKD development. In females, post-menopause lack of estrogen supply has been described as a risk factor for CKD development, and studies targeting post-menopause CKD are scarce. In the present study, we used exosomes isolated from bone marrow mesenchymal stem/stromal cells (BM-MSCs) to test their therapeutic potential against the development of CKD. At first, the menopause model was achieved by surgical bilateral ovariectomy in female albino rats. After that, 100 µg of exosomes was given to ovariectomized rats, and the study continued for 2 months. Changes in urine volume, urine protein content, kidney function biochemical parameters (creatinine and BUN), kidney antioxidant parameters (SOD, GPx and CAT), histological changes, immunohistochemical levels of caspase 3, and the gene expression of NGAL (related to kidney damage), TGFβ1 and αSMA (related to fibrosis and EMT), and caspase 3 (related to apoptosis) were studied. After the ovariectomy, the occurrence of CKD was confirmed in the rats by the drastic reduction of serum estrogen and progesterone levels, reduced urine output, increased urinary protein excretion, elevated serum creatinine and BUN, reduced GPx SOD, and CAT in kidney tissue, degenerative and fibrotic lesions in the histopathological examination, higher immunohistochemical expression of caspase 3 and increased expression of all studied genes. After exosomes administration, the entire chronic inflammatory picture in the kidney was corrected, and a near-normal kidney structure and function were attained. This study shows for the first time that BM-MSCs exosomes are potent for reducing apoptosis and fibrosis levels and, thus, can reduce the chronic damage of the kidneys in females that are in their menopause period. Therefore, MSCs-derived exosomes should be considered a valuable therapy for preserving postmenopausal kidney structure and function and, subsequently, could improve the quality of females' life during menopause.
Keywords: caspase 3; chronic renal injury; exosome therapy; fibrosis; nephroprotective; renoprotective.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

