Production of New Microbially Conjugated Bile Acids by Human Gut Microbiota
- PMID: 35625615
- PMCID: PMC9139144
- DOI: 10.3390/biom12050687
Production of New Microbially Conjugated Bile Acids by Human Gut Microbiota
Abstract
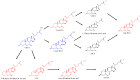
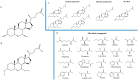
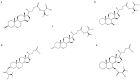
Gut microbes have been recognized to convert human bile acids by deconjugation, dehydroxylation, dehydrogenation, and epimerization of the cholesterol core, but the ability to re-conjugate them with amino acids as an additional conversion has been recently described. These new bile acids are known as microbially conjugated bile acids (MCBAs). The aim of this study was to evaluate the MCBAs diversity produced by the gut microbiota through a metabolomics approach. In this study, fresh fecal samples from healthy donors were evaluated to explore the re-conjugation of chenodeoxycholic and 3-oxo-chenodeoxycholic acids by the human gut microbiota. No significant differences were found between the conversion trend of both BAs incubations. The in vitro results showed a clear trend to first accumulate the epimer isoursochenodeoxycholic acid and the dehydroxylated lithocholic acid derivatives in samples incubated with chenodeoxycholic and 3-oxo-chenodeoxycholic acid. They also showed a strong trend for the production of microbially conjugated dehydroxylated bile acids instead of chenodeoxycholic backbone conjugates. Different molecules and isomers of MCBAs were identified, and the new ones, valolithocholate ester and leucolithocholate ester, were identified and confirmed by MS/MS. These results document the gut microbiota's capability to produce esters of MCBAs on hydroxyls of the sterol backbone in addition to amides at the C24 acyl site. This study opens a new perspective to study the BAs diversity produced by the human gut microbiota.
Keywords: MCBAs; bile acids; gut microbiota; metabolomics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Novel Insights into the Human Gut Microbially Conjugated Bile Acids: The New Diversity of the Amino Acid-Conjugated Derivatives.J Agric Food Chem. 2025 Aug 6;73(31):19460-19473. doi: 10.1021/acs.jafc.5c03548. Epub 2025 Jul 24. J Agric Food Chem. 2025. PMID: 40705851 Free PMC article.
-
Microbially catalyzed conjugation of GABA and tyramine to bile acids.J Bacteriol. 2024 Jan 25;206(1):e0042623. doi: 10.1128/jb.00426-23. Epub 2024 Jan 4. J Bacteriol. 2024. PMID: 38174933 Free PMC article.
-
Bile salt hydrolase acyltransferase activity expands bile acid diversity.Nature. 2024 Feb;626(8000):852-858. doi: 10.1038/s41586-024-07017-8. Epub 2024 Feb 7. Nature. 2024. PMID: 38326608
-
Review: microbial transformations of human bile acids.Microbiome. 2021 Jun 14;9(1):140. doi: 10.1186/s40168-021-01101-1. Microbiome. 2021. PMID: 34127070 Free PMC article. Review.
-
Bile acid and its bidirectional interactions with gut microbiota: a review.Crit Rev Microbiol. 2024 Sep;50(5):684-701. doi: 10.1080/1040841X.2023.2262020. Epub 2023 Sep 27. Crit Rev Microbiol. 2024. PMID: 37766478 Review.
Cited by
-
Electrostatic Interactions Dictate Bile Salt Hydrolase Substrate Preference.bioRxiv [Preprint]. 2023 Sep 26:2023.09.25.559308. doi: 10.1101/2023.09.25.559308. bioRxiv. 2023. Update in: Biochemistry. 2023 Nov 7;62(21):3076-3084. doi: 10.1021/acs.biochem.3c00210. PMID: 37808785 Free PMC article. Updated. Preprint.
-
Novel Insights into the Human Gut Microbially Conjugated Bile Acids: The New Diversity of the Amino Acid-Conjugated Derivatives.J Agric Food Chem. 2025 Aug 6;73(31):19460-19473. doi: 10.1021/acs.jafc.5c03548. Epub 2025 Jul 24. J Agric Food Chem. 2025. PMID: 40705851 Free PMC article.
-
Bile acids as inflammatory mediators and modulators of intestinal permeability.Front Immunol. 2022 Dec 7;13:1021924. doi: 10.3389/fimmu.2022.1021924. eCollection 2022. Front Immunol. 2022. PMID: 36569849 Free PMC article. Review.
-
Diagnosis by Microbial Culture, Breath Tests and Urinary Excretion Tests, and Treatments of Small Intestinal Bacterial Overgrowth.Antibiotics (Basel). 2023 Jan 28;12(2):263. doi: 10.3390/antibiotics12020263. Antibiotics (Basel). 2023. PMID: 36830173 Free PMC article. Review.
-
Novel microbial modifications of bile acids and their functional implications.Imeta. 2024 Oct 13;3(5):e243. doi: 10.1002/imt2.243. eCollection 2024 Oct. Imeta. 2024. PMID: 39429880 Free PMC article.
References
-
- Bjorkhem I., Araya Z., Rudling M., Angelin B., Einarsson C., Wikvall K. Differences in the regulation of the classical and the alternative pathway for bile acid synthesis in human liver—No coordinate regulation of CYP7A1 and CYP27A1. J. Biol. Chem. 2002;277:26804–26807. doi: 10.1074/jbc.M202343200. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

