Metformin Protects against Diabetic Cardiomyopathy: An Association between Desmin-Sarcomere Injury and the iNOS/mTOR/TIMP-1 Fibrosis Axis
- PMID: 35625721
- PMCID: PMC9139128
- DOI: 10.3390/biomedicines10050984
Metformin Protects against Diabetic Cardiomyopathy: An Association between Desmin-Sarcomere Injury and the iNOS/mTOR/TIMP-1 Fibrosis Axis
Abstract
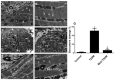
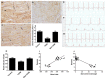
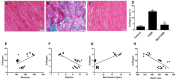
The intermediate filament protein desmin is essential for maintaining the structural integrity of sarcomeres, the fundamental unit of cardiac muscle. Diabetes mellitus (DM) can cause desmin to become dysregulated, following episodes of nitrosative stress, through the activation of the iNOS/mTOR/TIMP-1 pathway, thereby stimulating collagen deposition in the myocardium. In this study, type 2 diabetes mellitus (T2DM) was induced in rats. One group of animals was pre-treated with metformin (200 mg/kg) prior to diabetes induction and subsequently kept on metformin until sacrifice at week 12. Cardiac injuries developed in the diabetic rats as demonstrated by a significant (p < 0.0001) inhibition of desmin immunostaining, profound sarcomere ultrastructural alterations, substantial damage to the left ventricular tissue, collagen deposition, and abnormal ECG recordings. DM also significantly induced the cardiac expression of inducible nitric oxide synthase (iNOS), mammalian target of rapamycin (mTOR), and the profibrogenic biomarker tissue inhibitor of metalloproteinase-1 (TIMP-1). The expression of all these markers was significantly inhibited by metformin. In addition, a significant (p < 0.0001) correlation between desmin tissue levels/sarcomere damage and glycated hemoglobin, heart rate, iNOS, mTOR, and fibrosis was observed. These findings demonstrate an association between damage of the cardiac contractile unit—desmin and sarcomere—and the iNOS/mTOR/TIMP-1/collagen axis of fibrosis in T2DM-induced cardiomyopathy, with metformin exhibiting beneficial cardiovascular pleiotropic effects.
Keywords: cardiomyopathy; desmin; diabetes; fibrosis; metformin; sarcomere.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Danaei G., Finucane M.M., Lu Y., Singh G.M., Cowan M.J., Paciorek C.J. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: Systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet. 2011;378:31–40. doi: 10.1016/S0140-6736(11)60679-X. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

