Genetic Alterations in Benign Adrenal Tumors
- PMID: 35625779
- PMCID: PMC9138431
- DOI: 10.3390/biomedicines10051041
Genetic Alterations in Benign Adrenal Tumors
Abstract
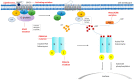
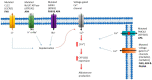
The genetic basis of most types of adrenal adenomas has been elucidated over the past decade, leading to the association of adrenal gland pathologies with specific molecular defects. Various genetic studies have established links between variants affecting the protein kinase A (PKA) signaling pathway and benign cortisol-producing adrenal lesions. Specifically, genetic alterations in GNAS, PRKAR1A, PRKACA, PRKACB, PDE11A, and PDE8B have been identified. The PKA signaling pathway was initially implicated in the pathogenesis of Cushing syndrome in studies aiming to understand the underlying genetic defects of the rare tumor predisposition syndromes, Carney complex, and McCune-Albright syndrome, both affected by the same pathway. In addition, germline variants in ARMC5 have been identified as a cause of primary bilateral macronodular adrenal hyperplasia. On the other hand, primary aldosteronism can be subclassified into aldosterone-producing adenomas and bilateral idiopathic hyperaldosteronism. Various genes have been reported as causative for benign aldosterone-producing adrenal lesions, including KCNJ5, CACNA1D, CACNA1H, CLCN2, ATP1A1, and ATP2B3. The majority of them encode ion channels or pumps, and genetic alterations lead to ion transport impairment and cell membrane depolarization which further increase aldosterone synthase transcription and aldosterone overproduction though activation of voltage-gated calcium channels and intracellular calcium signaling. In this work, we provide an overview of the genetic causes of benign adrenal tumors.
Keywords: Cushing syndrome; PKA; PRKAR1A; adrenal tumors; genetics.
Conflict of interest statement
C.A.S. holds patents on the PRKAR1A, PDE11A and GPR101 genes and/or their function and has received research funding from Pfizer Inc. on the genetics and treatment of abnormalities of growth hormone secretion.
Figures

References
-
- Grumbach M.M., Biller B.M., Braunstein G.D., Campbell K.K., Carney J.A., Godley P.A., Harris E.L., Lee J.K., Oertel Y.C., Posner M.C., et al. Management of the clinically inapparent adrenal mass (“incidentaloma”) Ann. Intern. Med. 2003;138:424–429. doi: 10.7326/0003-4819-138-5-200303040-00013. - DOI - PubMed
-
- Fassnacht M., Arlt W., Bancos I., Dralle H., Newell-Price J., Sahdev A., Tabarin A., Terzolo M., Tsagarakis S., Dekkers O.M. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur. J. Endocrinol. 2016;175:G1–G34. doi: 10.1530/EJE-16-0467. - DOI - PubMed
-
- Zeiger M.A., Thompson G.B., Duh Q.Y., Hamrahian A.H., Angelos P., Elaraj D., Fishman E., Kharlip J., Garber J.R., Mechanick J.I., et al. American Association of Clinical Endocrinologists and American Association of Endocrine Surgeons Medical Guidelines for the Management of Adrenal Incidentalomas: Executive summary of recommendations. Endocr. Pract. 2009;15:450–453. doi: 10.4158/EP.15.5.450. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

