Hypoxia Induces DPSC Differentiation versus a Neurogenic Phenotype by the Paracrine Mechanism
- PMID: 35625792
- PMCID: PMC9138575
- DOI: 10.3390/biomedicines10051056
Hypoxia Induces DPSC Differentiation versus a Neurogenic Phenotype by the Paracrine Mechanism
Abstract
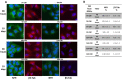
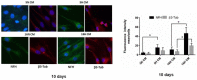
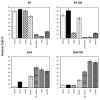
As previously described by several authors, dental pulp stem cells (DPSCs), when adequately stimulated, may acquire a neuronal-like phenotype acting as a favorable source of stem cells in the generation of nerves. Besides, it is known that hypoxia conditioning is capable of stimulating cell differentiation as well as survival and self-renewal, and that multiple growth factors, including Epidermal Growth factor (EGF) and basic fibroblast growth factor (bFGF), are often involved in the induction of the neuronal differentiation of progenitor cells. In this work, we investigated the role of hypoxia in the commitment of DPSCs into a neuronal phenotype. These cells were conditioned with hypoxia (O2 1%) for 5 and 16 days; subsequently, we analyzed the proliferation rate and morphology, and tested the cells for neural and stem markers. Moreover, we verified the possible autocrine/paracrine role of DPSCs in the induction of neural differentiation by comparing the secretome profile of the hypoxic and normoxic conditioned media (CM). Our results showed that the hypoxia-mediated DPSC differentiation was time dependent. Moreover, conditioned media (CM derived from DPSCs stimulated by hypoxia were able, in turn, to induce the neural differentiation of SH-SY5Y neuroblastoma cells and undifferentiated DPSCs. In conclusion, under the herein-mentioned conditions, hypoxia seems to favor the differentiation of DPSCs into neuron-like cells. In this way, we confirm the potential clinical utility of differentiated neuronal DPSCs, and we also suggest the even greater potential of CM-derived-hypoxic DPSCs that could more readily be used in regenerative therapies.
Keywords: DPSCs; dental pulp; hypoxia; neuronal differentiation; stem cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Diomede F., Fonticoli L., Marconi G.D., Della Rocca Y., Rajan T.S., Trubiani O., Murmura G., Pizzicannella J. Decellularized Dental Pulp, Extracellular Vesicles, and 5-Azacytidine: A New Tool for Endodontic Regeneration. Biomedicines. 2022;10:403. doi: 10.3390/biomedicines10020403. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

