Novel Biological Therapies for Severe Asthma Endotypes
- PMID: 35625801
- PMCID: PMC9138687
- DOI: 10.3390/biomedicines10051064
Novel Biological Therapies for Severe Asthma Endotypes
Abstract
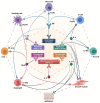
Severe asthma comprises several heterogeneous phenotypes, underpinned by complex pathomechanisms known as endotypes. The latter are driven by intercellular networks mediated by molecular components which can be targeted by specific monoclonal antibodies. With regard to the biological treatments of either allergic or non-allergic eosinophilic type 2 asthma, currently available antibodies are directed against immunoglobulins E (IgE), interleukin-5 (IL-5) and its receptor, the receptors of interleukins-4 (IL-4) and 13 (IL-13), as well as thymic stromal lymphopoietin (TSLP) and other alarmins. Among these therapeutic strategies, the best choice should be made according to the phenotypic/endotypic features of each patient with severe asthma, who can thus respond with significant clinical and functional improvements. Conversely, very poor options so far characterize the experimental pipelines referring to the perspective biological management of non-type 2 severe asthma, which thereby needs to be the focus of future thorough research.
Keywords: IgE; alarmins; monoclonal antibodies; pro-inflammatory cytokines; type 2 severe asthma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

