Toxic Exposure to Endocrine Disruptors Worsens Parkinson's Disease Progression through NRF2/HO-1 Alteration
- PMID: 35625810
- PMCID: PMC9138892
- DOI: 10.3390/biomedicines10051073
Toxic Exposure to Endocrine Disruptors Worsens Parkinson's Disease Progression through NRF2/HO-1 Alteration
Erratum in
-
Correction: D'Amico et al. Toxic Exposure to Endocrine Disruptors Worsens Parkinson's Disease Progression through NRF2/HO-1 Alteration. Biomedicines 2022, 10, 1073.Biomedicines. 2024 Apr 10;12(4):834. doi: 10.3390/biomedicines12040834. Biomedicines. 2024. PMID: 38672284 Free PMC article.
Abstract
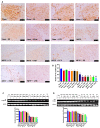
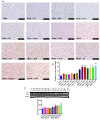
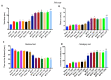
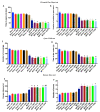
Human exposure to endocrine disruptors (EDs) has attracted considerable attention in recent years. Different studies showed that ED exposure may exacerbate the deterioration of the nervous system's dopaminergic capacity and cerebral inflammation, suggesting a promotion of neurodegeneration. In that regard, the aim of this research was to investigate the impact of ED exposure on the neuroinflammation and oxidative stress in an experimental model of Parkinson's disease (PD). PD was induced by intraperitoneally injections of MPTP for a total dose of 80 mg/kg for each mouse. Mice were orally exposed to EDs, starting 24 h after the first MPTP administration and continuing through seven additional days. Our results showed that ED exposure raised the loss of TH and DAT induced by the administration of MPTP, as well as increased aggregation of α-synuclein, a key marker of PD. Additionally, oral exposure to EDs induced astrocytes and microglia activation that, in turn, exacerbates oxidative stress, perturbs the Nrf2 signaling pathway and activates the cascade of MAPKs. Finally, we performed behavioral tests to demonstrate that the alterations in the dopaminergic system also reflected behavioral and cognitive alterations. Importantly, these changes are more significant after exposure to atrazine compared to other EDs. The results from our study provide evidence that exposure to EDs may play a role in the development of PD; therefore, exposure to EDs should be limited.
Keywords: Parkinson’s disease; atrazine; endocrine disruptors; inflammation; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- D’Amico R., Monaco F., Fusco R., Siracusa R., Impellizzeri D., Peritore A.F., Crupi R., Gugliandolo E., Cuzzocrea S., Di Paola R., et al. Atrazine Inhalation Worsen Pulmonary Fibrosis Regulating the Nuclear Factor-Erythroid 2-Related Factor (Nrf2) Pathways Inducing Brain Comorbidities. Cell. Physiol. Biochem. 2021;55:704–725. doi: 10.33594/000000471. - DOI - PubMed
-
- Genovese T., Siracusa R., Fusco R., D’Amico R., Impellizzeri D., Peritore A.F., Crupi R., Gugliandolo E., Morabito R., Cuzzocrea S., et al. Atrazine Inhalation Causes Neuroinflammation, Apoptosis and Accelerating Brain Aging. Int. J. Mol. Sci. 2021;22:7938. doi: 10.3390/ijms22157938. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

