Human iPSC Modeling of Genetic Febrile Seizure Reveals Aberrant Molecular and Physiological Features Underlying an Impaired Neuronal Activity
- PMID: 35625812
- PMCID: PMC9138645
- DOI: 10.3390/biomedicines10051075
Human iPSC Modeling of Genetic Febrile Seizure Reveals Aberrant Molecular and Physiological Features Underlying an Impaired Neuronal Activity
Abstract
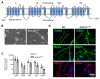
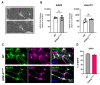
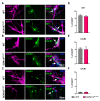
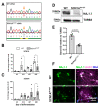
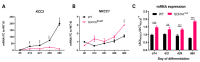
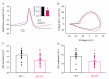
Mutations in SCN1A gene, encoding the voltage-gated sodium channel (VGSC) NaV1.1, are widely recognized as a leading cause of genetic febrile seizures (FS), due to the decrease in the Na+ current density, mainly affecting the inhibitory neuronal transmission. Here, we generated induced pluripotent stem cells (iPSCs)-derived neurons (idNs) from a patient belonging to a genetically well-characterized Italian family, carrying the c.434T > C mutation in SCN1A gene (hereafter SCN1AM145T). A side-by-side comparison of diseased and healthy idNs revealed an overall maturation delay of SCN1AM145T cells. Membranes isolated from both diseased and control idNs were injected into Xenopus oocytes and both GABA and AMPA currents were successfully recorded. Patch-clamp measurements on idNs revealed depolarized action potential for SCN1AM145T, suggesting a reduced excitability. Expression analyses of VGSCs and chloride co-transporters NKCC1 and KCC2 showed a cellular “dysmaturity” of mutated idNs, strengthened by the high expression of SCN3A, a more fetal-like VGSC isoform, and a high NKCC1/KCC2 ratio, in mutated cells. Overall, we provide strong evidence for an intrinsic cellular immaturity, underscoring the role of mutant NaV1.1 in the development of FS. Furthermore, our data are strengthening previous findings obtained using transfected cells and recordings on human slices, demonstrating that diseased idNs represent a powerful tool for personalized therapy and ex vivo drug screening for human epileptic disorders.
Keywords: disease model; febrile seizure; induced pluripotent stem cells; mesial temporal lobe epilepsy; voltage gated sodium channel NaV1.1.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Transcriptomic and electrophysiological alterations underlying phenotypic variability in SCN1A-associated febrile seizures.Sci Rep. 2025 Jul 10;15(1):24794. doi: 10.1038/s41598-025-09208-3. Sci Rep. 2025. PMID: 40634438 Free PMC article.
-
Hyperexcitability and Pharmacological Responsiveness of Cortical Neurons Derived from Human iPSCs Carrying Epilepsy-Associated Sodium Channel Nav1.2-L1342P Genetic Variant.J Neurosci. 2021 Dec 8;41(49):10194-10208. doi: 10.1523/JNEUROSCI.0564-21.2021. Epub 2021 Oct 29. J Neurosci. 2021. PMID: 34716231 Free PMC article.
-
Anomalous levels of Cl- transporters cause a decrease of GABAergic inhibition in human peritumoral epileptic cortex.Epilepsia. 2011 Sep;52(9):1635-44. doi: 10.1111/j.1528-1167.2011.03111.x. Epub 2011 Jun 2. Epilepsia. 2011. PMID: 21635237
-
NaV1.1 channels and epilepsy.J Physiol. 2010 Jun 1;588(Pt 11):1849-59. doi: 10.1113/jphysiol.2010.187484. Epub 2010 Mar 1. J Physiol. 2010. PMID: 20194124 Free PMC article. Review.
-
Model systems for studying cellular mechanisms of SCN1A-related epilepsy.J Neurophysiol. 2016 Apr;115(4):1755-66. doi: 10.1152/jn.00824.2015. Epub 2016 Feb 3. J Neurophysiol. 2016. PMID: 26843603 Free PMC article. Review.
Cited by
-
Modeling Sporadic Progressive Supranuclear Palsy in 3D Midbrain Organoids: Recapitulating Disease Features for In Vitro Diagnosis and Drug Discovery.Ann Neurol. 2025 May;97(5):845-859. doi: 10.1002/ana.27172. Epub 2025 Jan 28. Ann Neurol. 2025. PMID: 39876539 Free PMC article.
-
GABAA receptor function is enhanced by Interleukin-10 in human epileptogenic gangliogliomas and its effect is counteracted by Interleukin-1β.Sci Rep. 2022 Oct 26;12(1):17956. doi: 10.1038/s41598-022-22806-9. Sci Rep. 2022. PMID: 36289354 Free PMC article.
-
Transcriptomic and electrophysiological alterations underlying phenotypic variability in SCN1A-associated febrile seizures.Sci Rep. 2025 Jul 10;15(1):24794. doi: 10.1038/s41598-025-09208-3. Sci Rep. 2025. PMID: 40634438 Free PMC article.
-
The long-term neurodevelopmental outcomes of febrile seizures and underlying mechanisms.Front Cell Dev Biol. 2023 May 25;11:1186050. doi: 10.3389/fcell.2023.1186050. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37305674 Free PMC article. Review.
-
Insights into the Genetic Profile of Two Siblings Affected by Unverricht-Lundborg Disease Using Patient-Derived hiPSCs.Cells. 2022 Nov 4;11(21):3491. doi: 10.3390/cells11213491. Cells. 2022. PMID: 36359887 Free PMC article.
References
LinkOut - more resources
Full Text Sources

