Far-Infrared Therapy Decreases Orthotopic Allograft Transplantation Vasculopathy
- PMID: 35625826
- PMCID: PMC9139124
- DOI: 10.3390/biomedicines10051089
Far-Infrared Therapy Decreases Orthotopic Allograft Transplantation Vasculopathy
Abstract
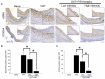
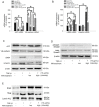
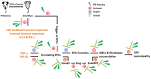
Orthotopic allograft transplantation (OAT) is a major strategy for solid heart and kidney failure. However, the recipient's immunity-induced chronic rejection induces OAT vasculopathy that results in donor organ failure. With the exception of immunosuppressive agents, there are currently no specific means to inhibit the occurrence of OAT vasculopathy. On the other hand, far-infrared (FIR) therapy uses low-power electromagnetic waves given by FIR, with a wavelength of 3-25 μm, to improve human physiological functions. Previous studies have shown that FIR therapy can effectively inhibit inflammation. It has also been widely used in adjuvant therapy for various clinical diseases, especially cardiovascular diseases, in recent years. Thus, we used this study to explore the feasibility of FIR in preventing OAT vasculopathy. In this study, the model of transplantation of an aorta graft from PVG/Seac rat to ACI/NKyo rat, and in vitro model of human endothelial progenitor cells (EPCs) was used. In this report, we presented that FIR therapy decreased the serious of vasculopathy in OAT-recipient ACI/NKyo rats via inhibiting proliferation of smooth muscle cells, accumulation of collagen, and infiltration of fibroblast in the vessel wall; humoral and cell-mediated immune responses were decreased in the spleen. The production of inflammatory proteins/cytokines also decreased in the plasma. Additionally, FIR therapy presented higher mobilization and circulating EPC levels associated with vessel repair in OAT-recipient ACI/NKyo rats. In vitro studies demonstrated that the underlying mechanisms of FIR therapy inhibiting OAT vasculopathy may be associated with the inhibition of the Smad2-Slug axis endothelial mesenchymal transition (EndoMT). Thus, FIR therapy may be the strategy to prevent chronic rejection-induced vasculopathy.
Keywords: endothelial mesenchymal transition; endothelial progenitor cells; far-infrared (FIR) therapy; orthotopic allograft transplantation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Sobajima M., Nozawa T., Ihori H., Shida T., Ohori T., Suzuki T., Matsuki A., Yasumura S., Inoue H. Repeated sauna therapy improves myocardial perfusion in patients with chronically occluded coronary artery-related ische-mia. Int. J. Cardiol. 2013;167:237–243. doi: 10.1016/j.ijcard.2011.12.064. - DOI - PubMed
-
- Lai C.C., Fang H.C., Mar G.Y., Liou J.C., Tseng C.J., Liu C.P. Post-angioplasty far infrared radiation therapy improves 1-year angioplasty-free hemodialysis access patency of recurrent obstructive lesions. Eur. J. Vasc. Endovasc. Surg. 2013;46:726–732. doi: 10.1016/j.ejvs.2013.09.018. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

