Natural Polymers in Heart Valve Tissue Engineering: Strategies, Advances and Challenges
- PMID: 35625830
- PMCID: PMC9139175
- DOI: 10.3390/biomedicines10051095
Natural Polymers in Heart Valve Tissue Engineering: Strategies, Advances and Challenges
Abstract
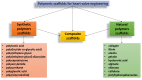
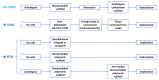
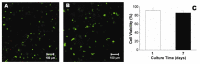
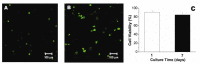
In the history of biomedicine and biomedical devices, heart valve manufacturing techniques have undergone a spectacular evolution. However, important limitations in the development and use of these devices are known and heart valve tissue engineering has proven to be the solution to the problems faced by mechanical and prosthetic valves. The new generation of heart valves developed by tissue engineering has the ability to repair, reshape and regenerate cardiac tissue. Achieving a sustainable and functional tissue-engineered heart valve (TEHV) requires deep understanding of the complex interactions that occur among valve cells, the extracellular matrix (ECM) and the mechanical environment. Starting from this idea, the review presents a comprehensive overview related not only to the structural components of the heart valve, such as cells sources, potential materials and scaffolds fabrication, but also to the advances in the development of heart valve replacements. The focus of the review is on the recent achievements concerning the utilization of natural polymers (polysaccharides and proteins) in TEHV; thus, their extensive presentation is provided. In addition, the technological progresses in heart valve tissue engineering (HVTE) are shown, with several inherent challenges and limitations. The available strategies to design, validate and remodel heart valves are discussed in depth by a comparative analysis of in vitro, in vivo (pre-clinical models) and in situ (clinical translation) tissue engineering studies.
Keywords: heart valve replacement; heart valve tissue engineering; polysaccharides; proteins; regenerative medicine; scaffold.
Conflict of interest statement
The authors declare no conflict of interest.
Figures















Similar articles
-
Tri-layered elastomeric scaffolds for engineering heart valve leaflets.Biomaterials. 2014 Sep;35(27):7774-85. doi: 10.1016/j.biomaterials.2014.04.039. Epub 2014 Jun 16. Biomaterials. 2014. PMID: 24947233 Free PMC article.
-
Biodegradable and biomimetic elastomeric scaffolds for tissue-engineered heart valves.Acta Biomater. 2017 Jan 15;48:2-19. doi: 10.1016/j.actbio.2016.10.032. Epub 2016 Oct 22. Acta Biomater. 2017. PMID: 27780764 Review.
-
Bioengineering challenges for heart valve tissue engineering.Annu Rev Biomed Eng. 2009;11:289-313. doi: 10.1146/annurev-bioeng-061008-124903. Annu Rev Biomed Eng. 2009. PMID: 19413511 Review.
-
Engineering of a polymer layered bio-hybrid heart valve scaffold.Mater Sci Eng C Mater Biol Appl. 2015 Jun;51:263-73. doi: 10.1016/j.msec.2015.03.009. Epub 2015 Mar 11. Mater Sci Eng C Mater Biol Appl. 2015. PMID: 25842134
-
Macrophage-extracellular matrix interactions: Perspectives for tissue engineered heart valve remodeling.Front Cardiovasc Med. 2022 Sep 13;9:952178. doi: 10.3389/fcvm.2022.952178. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36176991 Free PMC article. Review.
Cited by
-
Mechanistic Insights into Bioprosthetic Heart Valve Calcification and Anti-Calcification Strategies.Rev Cardiovasc Med. 2025 May 20;26(5):36688. doi: 10.31083/RCM36688. eCollection 2025 May. Rev Cardiovasc Med. 2025. PMID: 40475731 Free PMC article. Review.
-
Tertiary prevention and treatment of rheumatic heart disease: a National Heart, Lung, and Blood Institute working group summary.BMJ Glob Health. 2023 Oct;8(Suppl 9):e012355. doi: 10.1136/bmjgh-2023-012355. BMJ Glob Health. 2023. PMID: 37914182 Free PMC article. Review.
-
Natural and Synthetic Polymers for Biomedical and Environmental Applications.Polymers (Basel). 2024 Apr 20;16(8):1159. doi: 10.3390/polym16081159. Polymers (Basel). 2024. PMID: 38675078 Free PMC article. Review.
-
Bioprinting approaches in cardiac tissue engineering to reproduce blood-pumping heart function.iScience. 2024 Dec 20;28(1):111664. doi: 10.1016/j.isci.2024.111664. eCollection 2025 Jan 17. iScience. 2024. PMID: 39868032 Free PMC article. Review.
-
Recent advancements in polymeric heart valves: From basic research to clinical trials.Mater Today Bio. 2024 Aug 10;28:101194. doi: 10.1016/j.mtbio.2024.101194. eCollection 2024 Oct. Mater Today Bio. 2024. PMID: 39221196 Free PMC article. Review.
References
-
- Peeters F.E.C.M., Meex S.J.R., Dweck M.R., Aikawa E., Crijns H.J.G.M., Schurgers L.J., Kietselaer B.L.J.H. Calcific aortic valve stenosis: Hard disease in the heart: A biomolecular approach towards diagnosis and treatment. Eur. Heart J. 2018;39:2618–2624. doi: 10.1093/eurheartj/ehx653. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

